ICU Management & Practice, Volume 16 - Issue 4, 2016
Why Personalize Nutrition Therapy?
The need for personalized nutrition therapy for ICU patients is shown by several observational studies that measured the energy needs of critically ill patients. The 2005 study by Villet and colleagues found that patients with an energy deficit had an increased number of complications, especially infections (Villet et al. 2005). Weijs and colleagues (2014) showed in a cohort of 843 patients that survival varied according to the energy deficit; with no energy deficit there was a high rate of survival, but with a certain energy deficit a low rate of survival. In non-septic critically ill patients, early high protein intake was associated with lower mortality and early energy overfeeding with higher mortality. In septic patients early high protein intake had no beneficial effect on mortality. The study by Krishnan and colleagues showed that a moderate caloric intake of approximately 9 to 18 kcal/kg per day was associated with better outcomes than higher levels of caloric intake, and yet this was below the American College of Chest Physicians’ recommendations (Krishnan et al. 2003).
The hypothesis that hypocaloric feeding is beneficial is summarized in a recent review of randomized controlled trials comparing standard amounts of enteral nutrition with lesser amounts (Koretz 2016), with varied outcomes. The study by Petros and colleagues is a small study (n=100) that showed hypocaloric feeding to be associated with more nosocomial infections but with more glucose control and less gastrointestinal intolerance. We are still waiting for conclusive data on hypocaloric feeding, however.
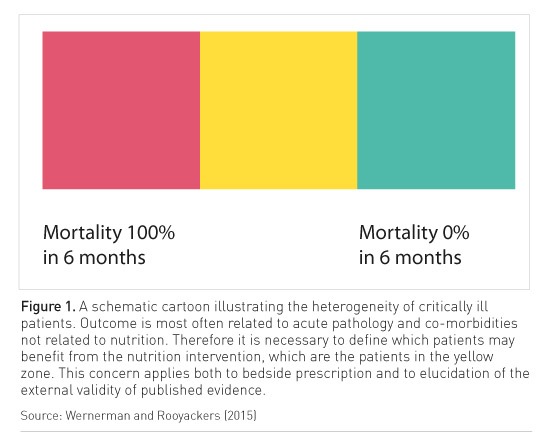
Surveys show that there is a difference between what nutrition is prescribed and what the patient actually receives (for example, Alberda et al. (2009) showed that patients received approximately half of what was prescribed). It seems that we do practise hypocaloric nutrition. In the ICU there will be a proportion of patients with a high risk of mortality, in whom nutrition is not likely to change the course of the illness. At the other extreme are the patients who will do well, who have a short stay in the ICU (Figure 1). Then are the others for whom nutrition is very important. But if we included all these groups of patients in a nutrition study, the results would be distorted. That is why many studies are inconclusive, as they do not have clearly defined inclusion criteria.
In ICU patients who receive nutrition there is basal energy expenditure, diet-induced energy expenditure, as we feed the patient, and activity-induced energy expenditure, as we try to mobilise patients. There is exogenous energy intake and the question is if this exogenous energy intake can completely eliminate mobilisation from endogenous stores. Very little is known about this, and there are good examples that it is the case that we cannot completely counteract mobilisation of energy inside the body. It is an important research question, as it relates to whether the energy expenditure we measure is always synonymous with caloric need. We know that we lose muscle regardless of what we do, because of inactivity and allergic reactions. There is much evidence that if you overfeed ICU patients, they are quite capable of having their body fat stores expanded by nutrition. There is a consensus not to overfeed patients, but not on how to define this, and whether energy expenditure is the correct parameter or not.
Guidelines
Recommend Indirect Calorimetry
The European and North American nutrition guidelines both recommend the use of indirect calorimetry to measure energy expenditures (Singer et al. 2009; McClave et al. 2016). The European guidelines state that during acute illness, the aim should be to provide energy as close as possible to the measured energy expenditure in order to decrease negative energy balance, and there is a recommendation for parenteral nutrition if indirect calorimetry is not used (Singer et al. 2009). The North American guidelines suggest use of indirect calorimetry (IC) to measure energy requirements, in the absence of variables that affect the accuracy of measurement (McClave et al. 2009).
There are arguments heard against indirect calorimetry—that it is expensive, inexact, technically difficult and time-consuming. It is not easy to interpret the data you get in all cases, but measurement is better than guesswork, and nothing is easy in the ICU. To sustain a correct nutrition plan we need the correct data. When continuous indirect calorimetry measurements were compared with formulas used to predict energy expenditure, they were better (Reid et al. 2007). It can be difficult to interpret, depending on the conditions. However, for patients at either extreme of body mass index (BMI), estimation with formulas is very difficult, and indirect calorimetry is the best tool. Indirect calorimetry should be used regularly because there is a learning curve and if it is not used regularly readings may be less reliable (Wernerman and Rooyackers 2015). The greatest difficulty in my view is to have a fair estimate of endogonous energy production that we cannot eliminate by exogenous energy production. And this is not a constant measurement and should therefore be repeated later in the ICU stay.
Indirect calorimetry is not time-consuming. Taking indirect calorimetry measurements for 15 minutes under standardised conditions is usually sufficient to measure energy expenditure. Zijlstra et al. (2007) showed that in their study that took measurements over 24 hours. If the patient has a long stay in the ICU, their energy expenditure will vary a lot, so measurements have to be taken on different days.
Most instruments for indirect calorimetry have sampling close to the patient and they have a flow meter that measures breath by breath. The International Multicentric Study Group for Indirect Calorimetry explored the issues with measurement for patients on mechanical ventilation; there are some technical difficulties in this as temperature and humidity must be measured (Oshima et al. 2016). Our ICU Metabolism and Nutrition research group at Karolinska Institute has published studies that compared indirect calorimetry instruments, and they compare quite well, with a scatter that, though not ideal, is better than using a formula or some other method of estimating energy expenditure (Sundström et al. 2013; Sundström Rehal et al. 2016).
Indirect calorimetry is integrated on a monitor or on a ventilator, and it does not need to be purchased separately. You should measure the cost of the device against the number of measurements it will take. Indirect calorimetry is not expensive when you consider that most of the ICU costs are staffing costs.
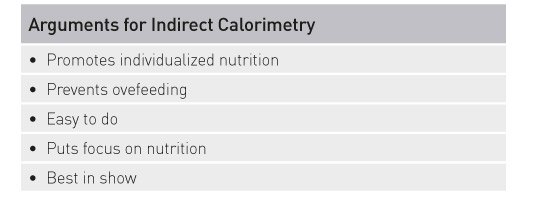
The most compelling argument for indirect calorimetry is that if you want to individualize nutrition for your patients, then you have to measure energy expenditure. Use of indirect calorimetry means there is a large scatter in relation to body size that clinicians need to be aware of. However, indirect calorimetry is an instrument to prevent overfeeding, it is easy to use, and it puts the right focus on nutrition. It is the “best in show”.
Take Home Points
- Indirect calorimetry (IC) is the gold standard to assess
the energy requirements of patients
- 15 minutes of indirect calorimetry under standardised
conditions is sufficient time to measure energy expenditure
- IC is available integrated into monitors or ventilators so
technically easy to measure and not an expensive add-on
- The best measurement we have right now
- No more difficult to interpret than many other measures
References:
Alberda C, Gramlich L, Jones N et al. (2009) The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med, 35(10): 1728-37. Erratum in: Intensive Care Med, 35(10): 1821.
Koretz RL (2016) JPEN Journal Club 22. Superiority, noninferiority, and equivalence. JPEN J Parenter Enteral Nutr, 40(7): 1064-6.
Krishnan JA, Parce PB, Martinez A et al. (2003) Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest, 124(1): 297-305.
McClave SA, Taylor BE, Martindale RG et al. (2016) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med, 44(2): 390-438.
Oshima T, Berger MM, De Waele E et al. (2016) Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr, Jun 22. pii: S0261-5614(16)30142-X. doi: 10.1016/j.clnu.2016.06.010. [Epub ahead of print]
Petros S, Horbach M, Seidel F et al. (2016) Hypocaloric vs Normocaloric Nutrition in Critically Ill Patients: A Prospective Randomized Pilot Trial. JPEN J Parenter Enteral Nutr, 40(2): 242-9.
Reid CL (2007) Poor agreement between continuous measurements of energy expenditure and routinely used prediction equations in intensive care unit patients. Clin Nutr, 26(5): 649-57.
Singer P, Berger MM, Van den Berghe Get al. (2009) ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr, 28(4): 387-400.
Sundström M, Tjäder I, Rooyackers O, Wernerman J (2013) Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr, 32(1): 118-21.
Sundström Rehal M, Fiskaare E, Tjäder I, Norberg Å, Rooyackers O, Wernerman J (2016) Measuring energy expenditure in the intensive care unit: a comparison of indirect calorimetry by E-sCOVX and Quark RMR with Deltatrac II in mechanically ventilated critically ill patients. Crit Care, 20:54.
Villet S, Chiolero RL, Bollmann MD et al. (2005) Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr, 24(4): 502-9.
Weijs PJ, Looijaard WG, Beishuizen A et al. (2014) Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care, 18(6): 701.
Wernerman J, Rooyackers O (2015) Nutrition monitoring. ICU Management, 15(3): 118,120,122.
Zijlstra N, ten Dam SM, Hulshof PJ et al. (2007) 24-hour indirect calorimetry in mechanically ventilated critically ill patients. Nutr Clin Pract, 22(2): 250-5