ICU Management & Practice, ICU Volume 12 - Issue 2 - Summer 2012
(Part II): results from a meta-analysis and practical approach
Co-authors
Colin Cordemans, MD
Intensive Care Unit
ZNA Stuivenberg
Antwerp, Belgium
Intensive Care Unit
ZNA Stuivenberg
Antwerp, Belgium
Introduction
In a previous issue of ICU Management (volume 12, issue 1), we suggested a three hit model of shock and emphasised that both early and late fluid management affect outcome in acute lung injury (ALI), sepsis and trauma (Rivers 2006; Bagshaw et al. 2008; Murphy et al. 2009; Prowle et al. 2010; Schrier 2010; Malbrain and Van Regenmortel 2012). After the initial adequate-filling phase to reverse distributive shock (Rivers et al. 2001), emphasis shifts to limitation and elimination of interstitial oedema in vital organs. Indeed, positive fluid balance resulting from third spacing is independently associated with impaired organ function and worse outcome, (Sakr et al. 2005; Malbrain et al. 2006; Vincent et al. 2006; Payen et al. 2008; Rosenberg et al. 2009). This was recently shown in a sophisticated retrospective study by Murphy and coworkers (Murphy et al. 2009). Conversely, achievement of negative fluid balance suggests survival and improved lung function (Alsous et al. 2000; Wiedemann et al. 2006). These have been referred to as the ebb and flow phases of shock.
The ebb phase was characterised by Cuthbertson in 1932, by the presence of “ashen faces, a thready pulse and cold clammy extremities”, while during the flow phase, “the patient warms up, cardiac output increases and the surgical team relaxes” (Malbrain et al. 2012). Recent data tell us, however, that many patients do not enter the flow phase spontaneously. In order to avoid a positive cumulative fluid balance with peripheral oedema and organ oedema, resulting in end-organ dysfunction and failure, these patients may need a little help in the transition from ebb to flow (Cordemans et al. 2012). This article will check the evidence with regard to the impact of a positive fluid balance on patient outcome and how to deal with it at the bedside.
Background and Results of Meta-Analysis
The below PICO method was used in performing a meta-analysis of the available literature in order to find an answer to the following question: Does a management strategy attempting to obtain a daily fluid balance (FB) close to zero, or even negative (conservative fluid strategy), after day three result in a lower intra-abdominal pressure (IAP) and improved patient outcome compared with management approach that accept a liberal fluid strategy, and will the latter result in higher IAPs in critically ill adults in intensive care units? 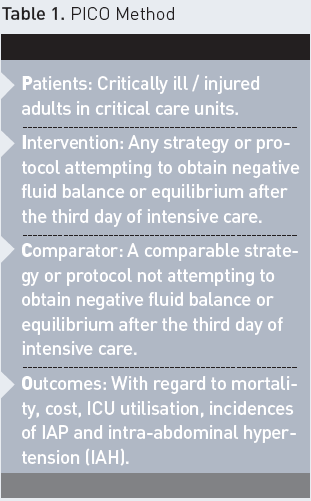
We applied the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system to guide the assessment of evidence when addressing the clinical management questions. Opinions were graded from high to very low to help determine the strength of recommendations. Proposals at the top of the scale (Grade 1A) suggested that the overall desirable effects of intervention clearly outweighed potential undesirable effects, while weaker recommendations (Grade 2D) indicated that the balance of risks and benefits for any intervention was rather unclear. Lowest ranking opinions suggested that clear uncertainty existed on any benefits from intervention, meaning that no final recommendation could be made.
By performing a Medline and Pubmed search, 40 articles were identified and included in this analysis: there was one meta-analysis (albeit only published in abstract form), while there were 10 randomised controlled clinical trials (four of which were blinded), seven interventional studies, 28 observational studies, and four case series. In total, 23,625 critically ill patients were studied in the 40 articles; in 23 studies the IAP was also measured. Through analysing the data collected, we developed four subquestions:
1. Do non-survivors have a more positive FB? Data on 3,246 patients from 13 studies showed, indeed, that non-survivors (n= 1,643, mortality being 50.6 percent) have a more positive cumulative FB by day seven of their ICU stay. The cumulative FB was on average 4,628 ml more positive in non-survivors compared to survivors. The summary of findings of these studies is given in Table 1.
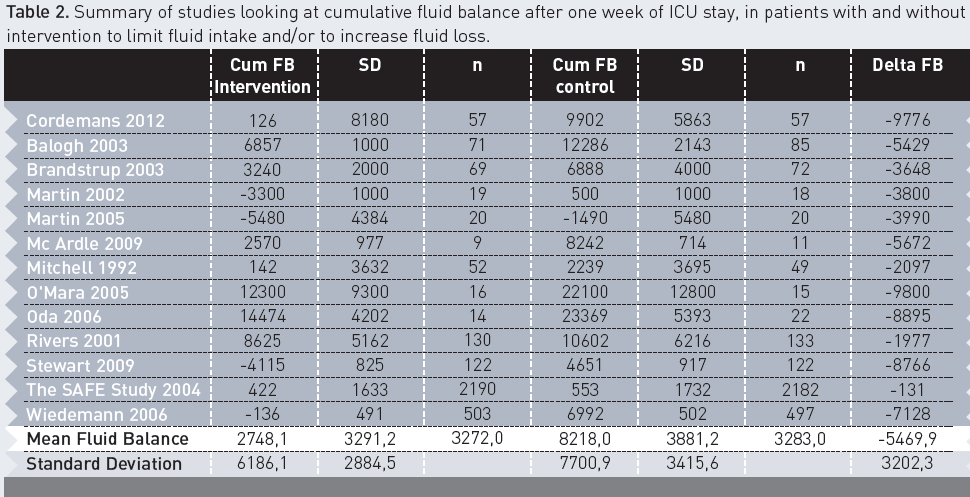
2. Does intervention to limit fluid intake or lower FB improve outcome? Data on 12,871 patients from 23 studies showed that outcome significantly improves with a conservative fluid regimen (odds ratio 0.34). In patients treated with a restrictive fluid regimen, mortality decreased from 29.1 percent (1,859 deaths in 6,384 patients) to 22.2 percent (1,443 deaths in 6,488 patients) (p < 0.0001). Actual data on cumulative FB was available in 6,555 patients from 13 studies. Data suggests that conservative treatment results in a less positive FB. Cumulative FB was on average 5,470 ml less positive after one week of ICU stay. The summary of findings of these studies is given in Table 2.
3. Do patients with IAH have a more positive FB? Data on 1,517 patients from eight studies showed that the 597 patients with IAH (incidence being 39.4 percent) had a more positive FB. The cumulative FB after one week of ICU stay was on average 3,389 ml more positive.
4. Does IAP improve with interventions acting on lowering FB? Only 10 studies looked at the effects of fluid removal (by using furosemide or renal replacement therapy with net ultrafiltration) on IAP. These were case studies or small series. A total fluid removal of 6,810 ml resulted in a drop in IAP from 21.5 mmHg to 12 mmHg. A dose related effect was observed: the more negative the FB, the greater the decrease in IAP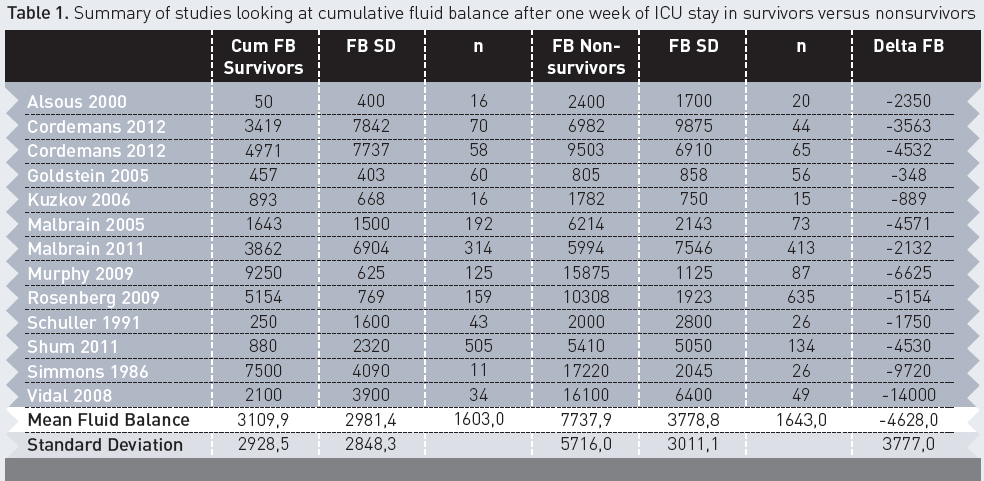
Suggestions for Management and a Practical Approach
In light of this evidence, we recommend the use of a protocol to try to avoid a positive cumulative FB occurring in the critically ill, especially in those with, or at risk of, IAH, and after acute resuscitation has been completed and the inciting issues and source control have been addressed (Grade 1B). We suggest obtaining a zero to negative FB by day three and keeping the cumulative FB on day seven as low as possible (Grade 1B). A vicious cycle leading to more fluid loading and further IAP increase is illustrated in Figure 1, and this must be avoided.
After reviewing the scarce evidence, we can only make a weak suggestion regarding the use of diuretics or renal replacement therapy (in combination with albumin) versus nothing to mobilise fluids in haemodynamically stable patients with IAH and a positive cumulative FB following acute resuscitation and source control measures (Grade 2C). The lack of consensus for this intervention underscores the uncertainity regarding it’s role in managing FB and subsequently IAH, and signifies the need for further study. 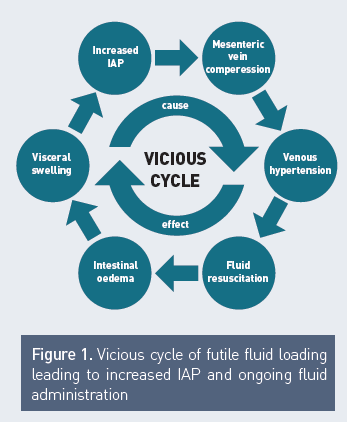
In addition to measuring extravascular lung water index (EVLWI), transcardiopulmonary thermodilution allows the extent of capillary leak and fluid overload to be estimated. Accordingly, EVLWI correlates well with organ function and survival (Sakka et al. 2002; Martin et al. 2005; Kuzkov et al. 2006; Phillips et al. 2008). Moreover, fluid management aimed at EVLWI reduction results in a more negative FB and improved outcomes (Mitchell et al. 1992). In order to achieve a negative FB, previous prospective trials excluded patients with hypotension and renal failure (Mitchell et al. 1992; Martin et al. 2005; Wiedemann et al. 2006).
Recently, the effects of a restrictive fluid regimen on patients with negative FB, who are using PAL-treatment, were examined in mechanically ventilated patients with ALI, who were presenting with severe hypoxemia, increased EVLWI and IAP (Cordemans et al. 2012). PAL-treatment combines high levels of positive end-expiratory pressure (PEEP), small volume resuscitation with hyperoncotic albumin, and fluid removal with diuretics or ultrafiltration during continuous renal replacement therapy (CRRT). First, a 30 minute application of PEEP is titrated to counterbalance the effects of increased IAP (best PEEP in cmH2O = IAP in mmHg). Next, hyperoncotic albumin (20 percent) solution is administered with 200 ml boluses, over a 60 minute period, twice on the first day; it is subsequently titrated towards a serum albumin level of 30 g/dl. Finally, after 30 minutes, a furosemide drip is initiated with an intravenous loading dose of 60 mg, followed by a continuous infusion at 60 mg per hour for the first 4 hours and 5-10 mg per hour thereafter, according to haemodynamic tolerance. In anuric patients, CRRT is initiated with an ultrafiltration rate resulting in neutral to negative daily fluid balances.
In a recent study of 57 patients, compared with 57 matched controls, we found significant beneficial effects of PAL-treatment on EVLWI, IAP, organ function and vasopressor therapy during one week. This resulted in a shorter duration of mechanical ventilation and an improved 28-day mortality. Through combining the results of two recent studies (with number of patients totalling 180), we found that the group of patients treated with conservative initial and late fluid management had the best outcome, followed by those who received initial adequate and late conservative fluid management (Cordemans et al. 2012; Cordemans et al. 2012). Mortality was significantly increased in those patients who received late liberal fluid management (Figure 2). This is in line with previous results by Murphy and co-workers (Murphy et al. 2009). 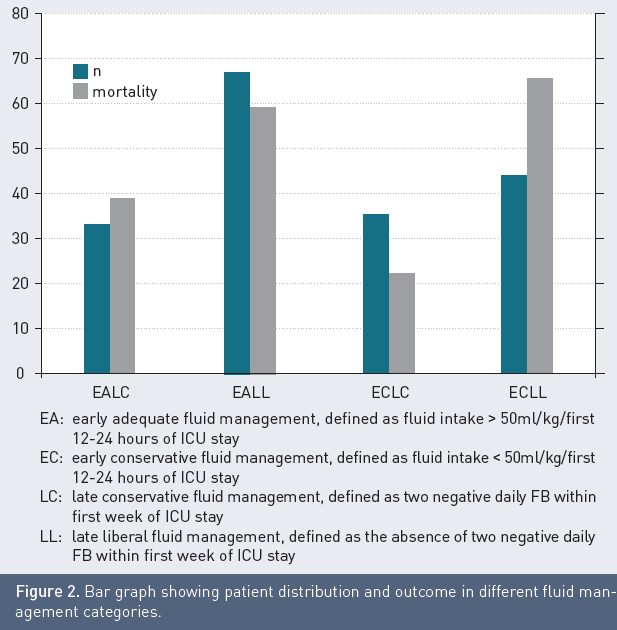
Key Messages
Late conservative fluid management may, in the long run, be even more important than initial resuscitation efforts during the ebb phase in patients with shock. EVLWI can be used at the bedside as a guide to initiating a conservative late fluid strategy or late goal directed fluid removal in those patients that do not transgress spontaneously from the ebb to the flow phase of shock. However, we must remember that no single parameter can change outcome; this can only be achieved by a good protocol. PAL-treatment seems a good example of such a protocol, but further prospective studies are needed.
References:
Bagshaw, S. M., P. D. Brophy, et al. (2008). "Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury." Crit Care 12(4): 169.
Beekley, A. C. (2008). "Damage control resuscitation: a sensible approach to the exsanguinating surgical patient." Critical care medicine 36(7 Suppl): S267-274.
Cordemans, C., I. De laet, et al. (2012). "Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak and fluid balance." Annals Intensive Care 2(Supplem 1): in press.
Cordemans, C., I. De laet, et al. (2012). "Aiming for negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: A pilot study looking at the effects of PAL-treatment." Annals Intensive Care 2(Supplem 1): in press.
Cotton, B. A., B. K. Au, et al. (2009). "Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications." J Trauma 66(1): 41-48; discussion 48-49.
Cotton, B. A., N. Reddy, et al. (2011). "Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients." Annals of surgery 254(4): 598-605.
Duchesne, J. C., J. M. Barbeau, et al. (2011). "Damage control resuscitation: from emergency department to the operating room." The American surgeon 77(2): 201-206.
Holcomb, J. B., D. Jenkins, et al. (2007). "Damage control resuscitation: directly addressing the early coagulopathy of trauma." J Trauma 62(2): 307-310.
Kuzkov, V. V., M. Y. Kirov, et al. (2006). "Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury." Crit Care Med 34(6): 1647-1653.
Malbrain, M. L., M. L. Cheatham, et al. (2006). "Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions." Intensive Care Med 32(11): 1722-1732.
Malbrain, M. L. N. G. and N. Van Regenmortel (2012). "Fluid overload is not only of cosmetic concern: Exploring a new hypothesis." ICU Management 12(1): 30-33.
Martin, G. S., S. Eaton, et al. (2005). "Extravascular lung water in patients with severe sepsis: a prospective cohort study." Crit Care 9(2): R74-82.
Martin, G. S., M. Moss, et al. (2005). "A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury." Crit Care Med 33(8): 1681-1687.
Mitchell, J. P., D. Schuller, et al. (1992). "Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization." Am Rev Respir Dis 145(5): 990-998.
Murphy, C. V., G. E. Schramm, et al. (2009). "The importance of fluid management in acute lung injury secondary to septic shock." Chest 136(1): 102-109.
Payen, D., A. C. de Pont, et al. (2008). "A positive fluid balance is associated with a worse outcome in patients with acute renal failure." Crit Care 12(3): R74.
Phillips, C. R., M. S. Chesnutt, et al. (2008). "Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival." Crit Care Med 36(1): 69-73.
Prowle, J. R., J. E. Echeverri, et al. (2010). "Fluid balance and acute kidney injury." Nat Rev Nephrol 6(2): 107-115.
Rivers, E., B. Nguyen, et al. (2001). "Early goal-directed therapy in the treatment of severe sepsis and septic shock." N Engl J Med 345(19): 1368-1377.
Rivers, E. P. (2006). "Fluid-management strategies in acute lung injury--liberal, conservative, or both?" N Engl J Med 354(24): 2598-2600.
Rosenberg, A. L., R. E. Dechert, et al. (2009). "Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort." J Intensive Care Med 24(1): 35-46.
Sakka, S. G., M. Klein, et al. (2002). "Prognostic value of extravascular lung water in critically ill patients." Chest 122(6): 2080-2086.
Sakr, Y., J. L. Vincent, et al. (2005). "High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury." Chest 128(5): 3098-3108.
Schrier, R. W. (2010). "Fluid administration in critically ill patients with acute kidney injury." Clin J Am Soc Nephrol 5(4): 733-739.
Vincent, J. L., Y. Sakr, et al. (2006). "Sepsis in European intensive care units: results of the SOAP study." Crit Care Med 34(2): 344-353.
Wiedemann, H. P., A. P. Wheeler, et al. (2006). "Comparison of two fluid-management strategies in acute lung injury." N Engl J Med 354(24): 2564-2575.






