ICU Management & Practice, ICU Volume 8 - Issue 3 - Autumn 2008
Authors
Implementing evidence is the basis for improving organisation of care in the intensive care unit. As this can be a challenging task due to human and system barriers, we propose an innovative framework to facilitate knowledge translation at the bedside.
Background
The mission of an Intensive Care Unit (ICU) may vary according to the type of unit, hospital and healthcare system involved (Walter et al. 2008). However, the universal objectives for all ICUs remain the same and are as follows:- To provide compassionate and evidence-based care for critically ill patients and families,
- To optimise resource allocation and utilisation,
- To coordinate care and provide critical care services to hospital stakeholders, like the emergency room (ER), surgical and medical services,
- To provide regional critical care support for specialised services, like trauma or burns and
- To create, develop and translate knowledge, if the ICU belongs to an academic hospital.
The Challenge of the ICU Environment
Despite the wealth of evidence-based practice and the drive to improve organisation of care, failure to successfully implement protocols and guidelines in the ICU environment is frequently reported (Blackwood 2003; Ferrer et al. 2008; Ibrahim and Kollef 2001; Trujillo et al. 2008; Walter et al. 2008). This can be attributed to the high level of complexity of the patients and illnesses, as well as the model of care delivery, requiring highly trained caregivers to interact in a multifaceted way (Simpson and Doig 2007). The purpose of this article is to describe a framework we designed to better identify potential barriers to change and to develop targeted action plans.Methods
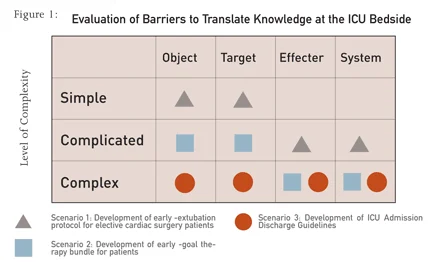
We propose a methodology to effectively implement protocols, guidelines or other kinds of change strategies within the ICU environment.For this purpose, we first identified that the failure to execute can be due to four categories of barriers found within the ICU setting [Fig1]:
- Complexity of information contained within protocols and guidelines that are often difficult to interpret in the local ICU environment and can contradict each other (Walter et al. 2008; Morris 2004; Morris 2003). In this paper, we will label this barrier as the “object”.
- Complexity and heterogeneity of ICU patients that often limit the translation of direct evidence from randomised controlled trials. Indeed, many of these randomised clinical trials exclude a large portion of severely ill and complex patients (Hammond 2001). We will label this barrier as the “target”.
- Complexity of change for the individual practitioner (-s) or healthcare team (-s), that will be the person (-s) required to implement this new evidence at the bedside. Cultural, interpersonal, and other change barriers fall into this category (Walter et al. 2008; Sinuff et al. 2007). We will label this barrier as the “effecter”.
- Complexity of the model of healthcare delivery in which the new evidence will be implemented. Variance in complexity will be observed, dependent on the level of formal organisation of the health system (MacKenzie et al. 2006). We will label this barrier as the “system”.
Each of these types of barriers has already been reported in the literature (Simpson and Doig 2008). However, a systematic framework that defines how one could tangibly overcome these obstacles in an articulated manner has not been described, as yet
. Second, the level of complexity of each of the four barriers is assessed and then categorized using a semi-quantitative tool developed by Westley and Zimmerman (Westley et al. 2007; Zimmerman et al. 1998). This tool permits the level of complexity to be evaluated as “simple”, “complicated” or “complex”.
We then assess and categorise each scenario using a matrix approach [Fig1]. Each barrier is listed and in terms of one of the three levels of complexity. When this level has been determined, an action plan can be developed to deal with the most complex barriers and assign resources accordingly.
Lastly, the action plan is then targeted to simplify the obstacles through communication, education and management techniques, while using a problem-solving approach. The goal of this methodology is to achieve successful knowledge translation of evidencebased care at the bedside using a project management methodology.
Results
We will describe three scenarios to illustrate this concept Scenario 1: Implementation of an Evidencebased Early Extubation Protocol in the Cardiac Surgery ICU for Elective Cardiac Surgery Patients.
Scenario 2: Development and Implementation of a Sepsis Bundle in the Medical Surgical ICU and ER.
Scenario 3: Development and Implementation of Admission and Discharge Guidelines for ICU Patients.
Conclusions
When implementing evidence at the bedside, our framework can help to optimise the organisation of care in the ICU. The methodology allows for the assessment of the complexity of the task, the detection of major obstacles and can guide the team’s action plan. We recommend use of the tool prospectively to plan for implementing new protocols and guidelines. In addition, the tool can be used retrospectively, to detect the root cause of failure and at the same time, allow for alternative solutions. It can also facilitate organisational projects such as patient flow management, academic program development, teamwork training and response to disasters and pandemics.Note of Appreciation
We would like to extend our thanks to the work of Dr. Brenda Zimmerman, Associate Professor, Schulich School of Business at York University in Toronto, Canada.