ICU Management & Practice, ICU Volume 15 - Issue 4 - 2015
- Delirium (acute brain dysfunction) can be a complication of critical illness.
- Brain organ dysfunction can manifest as a continuum of psychomotor behaviors that are categorised as hyperactive or hypoactive.
- Delirium can be diagnosed using validated and reliable bedside tools.
- Implementation of delirium monitoring can be enhanced by scheduled in-depth discussions about brain organ dysfunction via multidisciplinary rounds with the medical team.
- Delirium may be managed with use of non-pharmacologic and, if necessary, pharmacologic interventions thereafter.
Acute brain dysfunction is common in the critical care setting, presenting as delirium or coma. Delirium is a clinical syndrome of brain dysfunction characterised by an acute change or fluctuating course of altered mental status, inattention, and a disturbance of consciousness, cognition, or perception, such that a patient’s ability to receive, process, store, and recall information is impaired (American Psychiatric Association 2013).
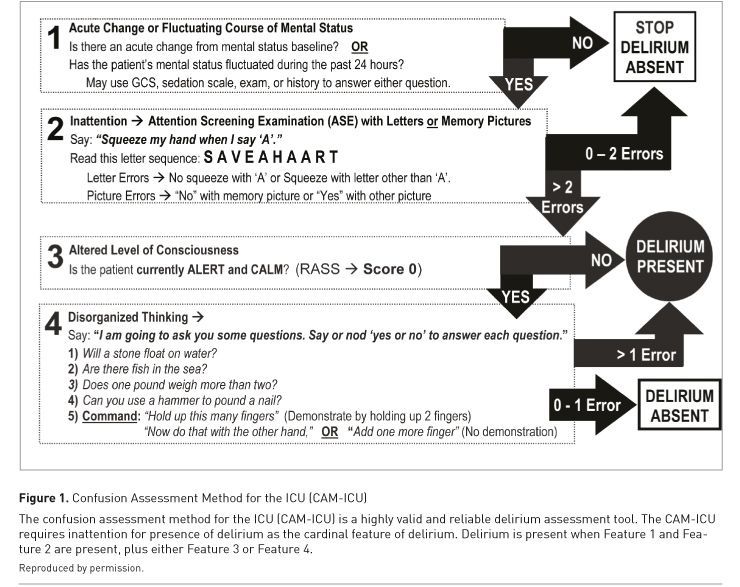
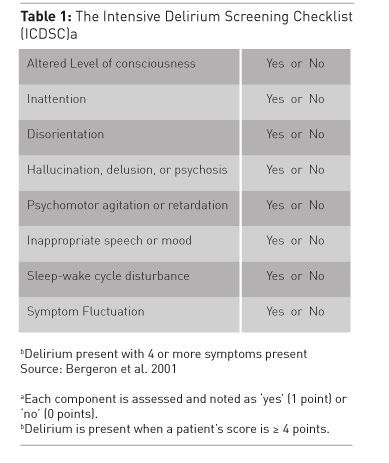
See Also: Sedation in Acute Brain Injury: Less is More?
References:
Adam N, Kandelman S, Mantz J et al. (2013) Sepsis-induced brain dysfunction. Expert Rev Anti Infect Ther, 11(2): 211-21.
Agarwal V, O'Neill PJ, Cotton BA et al. (2010) Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res, 31(5): 706-15.
American Psychiatric Association, ed. (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th edition. [Accessed: 9 November 2015] Available from dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596
Balas MC, Burke WJ, Gannon D et al. (2013) Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med, 41(9 Suppl 1): S116-27.
Barr J, Fraser GL, Puntillo K et al. (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit: executive summary. Am J Health Syst Pharm, 70(1): 53-8.
Bergeron N, Dubois MJ, Dumont M et al. (2001) Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med, 27(5): 859-64.
Brook AD, Ahrens TS, Schaiff R et al.(1999) Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med, 27(12): 2609-15.
Bryczkowski SB, Lopreiato MC, Yonclas PP et al.(2014) Risk factors for delirium in older trauma patients admitted to the surgical intensive care unit. J Trauma Acute Care Surg, 77(6): 944-51.
Colville G, Kerry S, Pierce C (2008) Children's factual and delusional memories of intensive care. Am J Respir Crit Care Med, 177(9): 976-82.
Devlin JW, Roberts RJ, Fong JJ et al. (2010) Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med, 38(2): 419-27.
Devlin JW, Skrobik Y, Riker RR et al. (2011) Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post-hoc analysis of a double-blind, randomized, placebo-controlled study. Crit Care, 15(5): R215.
Ely EW, Inouye SK, Bernard GR et al. (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA, 286(21): 2703-10.
Ely EW, Shintani A, Truman B et al. (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA, 291(14): 1753-62.
Ely EW, Gautam S, Margolin R et al. (2001b) The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med, 27(12): 1892-900.
Francis J, Martin D, Kapoor WN (1990) A prospective study of delirium in hospitalized elderly. JAMA, 263(8): 1097-101.
Fiser DH (1992) Assessing the outcome of pediatric intensive care. J Pediatr, 121(1): 68-74.
Girard TD, Kress JP, Fuchs BD et al. (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet, 371(9607): 126-34.
Girard TD, Pandharipande PP, Carson SS et al. (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med, 38(2): 428-37.
Goben C, Gangopadhyay M, Chestnut MH et al. (2014) Pediatric delirium prevalence and motoric subtypes in critically ill infants and young children. Crit Care Med, 2(12): A128.
Inouye SK, Charpentier PA (1996) Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA, 275(11): 852-7.
Inouye SK, Bogardus ST Jr., Charpentier PA et al. (1999) A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med, 340(9): 669-76.
Kamdar BB, Niessen T, Colantuoni E et al. (2015) Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med, 43(1):135-41.
Khan BA, Fadel WF, Tricker JL et al. (2014) Effectiveness of implementing a wake up and breathe program on sedation and delirium in the ICU. Crit Care Med, 42(12): e791-5.
Klein Klouwenberg PM, Zaal IJ, Spitoni C et al. (2014) The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ, 349: g6652.
Lat I, McMillian W, Taylor S et al. (2009) The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med, 37(6): 1898-905.
Leentjens AF, Schieveld JN, Leonard M et al. (2008) A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res, 64(2): 219-23.
Lin SM, Huang CD, Liu CY et al. (2008) Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care 2008, 23(3): 372-9.
McPherson JA, Wagner CE, Boehm LM et al. (2013) Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med, 41(2):405-13.
Meagher DJ, Moran M, Raju B et al. (2007) Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry, 190: 135-41.
Mehta S, Cook D, Devlin JW et al. (2015) Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med, 43(3): 557-66.
Page VJ, Ely EW, Gates S et al.(2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med, 1(7): 515-23.
Pandharipande P, Cotton BA, Shintani A et al. (2008) Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma, 65(1): 34-41.
Pandharipande P, Cotton BA, Shintani A et al. (2007) Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med, 33(10): 1726-31.
Pandharipande P, Jackson J, Ely EW (2005) Delirium: acute cognitive dysfunction in the critically ill. Curr Opin Crit Care, 11(4): 360-8.
Pandharipande P, Shintani A, Peterson J et al. (2006) Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology, 104(1): 21-6.
Pandharipande PP, Girard TD, Jackson JC et al. (2013) Long-term cognitive impairment after critical illness. N Engl J Med, 369(14): 1306-16.
Patel SB, Poston JT, Pohlman A et al. (2014) Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med, 189(6): 658-65.
Pauley E, Lishmanov A, Schumann S et al. (2015) Delirium is a robust predictor of morbidity and mortality among critically ill patients treated in the cardiac intensive care unit. Am Heart J, 170(1): 79-86.
Pun BT, Devlin JW (2013) Delirium monitoring in the ICU: strategies for initiating and sustaining screening efforts. Semin Respir Crit Care Med, 34(2): 179-88.
Rees G, Gledhill J, Garralda ME et al. (2004) Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med, 30(8): 1607-14.
Riker RR, Shehabi Y, Bokesch PM et al. (2009) Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA, 301(5): 489-99.
Schieveld JN, Leroy PL, Van OsJ et al. (2007) Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med, 33(6): 1033-40.
Schweickert WD, Pohlman MC, Pohlman AS et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet, 373(9678): 1874-82.
Sessler CN, Pedram S (2011) Protocolized and target-based sedation and analgesia in the ICU. Anesthesiol Clin, 29(4): 625-50.
Sevransky JE, Checkley W, Herrera P et al. (2015) Protocols and hospital mortality in critically ill patients: the United States critical illness and injury trials group critical illness outcomes study. Crit Care Med, Jun 24 [Epub ahead of print] doi: 10.1097/CCM.0000000000001157
Silver G, Traube C, Gerber L et al. (2015) Pediatric delirium and associated risk factors: a single-center prospective observational study. Ped Crit Care Med,16(4): 303-9.
Silver GH, Kearney JA, Kutko MC et al. (2010) Infant delirium in pediatric critical care settings. Am J Psychiatry, 167(10): 1172-7.
Skrobik YK, Bergeron N, Dumont M et al. (2004) Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med, 30(3): 444-9.
Smith HA, Boyd J, Fuchs DC et al. (2011) Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med, 39(1): 150-7.
Smith HAB, Gangopadhyay M, Goben C et al. (2014) Pediatric delirium in infants and young children: validation of the preschool confusion assessment method for the intensive care unit (psCAM-ICU). Am J Respir Crit Care Med, 189: A3246.
Smith HA, Brink E, Fuchs DC et al. (2013) Pediatric delirium: monitoring and management in the pediatric intensive care unit. Pediatr Clin North Am, 60(3): 741-60.
Traube C, Silver G, Kearney J et al. (2014) Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med, 42(3): 656-63.
Turkel SB, Trzepacz PT, Tavaré CJ (2006) Comparing symptoms of delirium in adults and children. Psychosomatics, 47(4): 320-4.
van den Boogaard M, Schoonhoven L, van AT et al. (2013) Haloperidol prophylaxis in critically ill patients with a high risk for delirium. Crit Care, 17(1): R9.
Zhang WY, Wu WL, Gu JJ et al. (2015) Risk factors for postoperative delirium in patients after coronary artery bypass grafting: A prospective cohort study. J Crit Care, 30(3): 606-12.








