ICU Management & Practice, ICU Volume 12 - Issue 1 - Spring 2012
Exploring a New Hypothesis
Introduction
Capillary leak complicates conditions that are characterised by inflammation, infection and resuscitation. It is important to understand the pathophysiologic basis in order to minimise fluid, electrolytes, proteins and other molecules that extravasate from the vascular space into the interstitium. Capillary leak not only contributes to peripheral but also organ oedema, as well as pleural effusions and ascites that accompany large volume resuscitation. As such, capillary leak significantly contributes to the genesis of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS). Recently, more evidence has become available, alluding to the deleterious effects of fluid accumulation. In this paper we hypothesise on an old theory related to this topic: the three hit model of shock (Malbrain and De Laet 2008).
Background
The concept of dual metabolic response to bodily injury was introduced as early as 1942. In direct response to initial proinflammatory cytokines and stress hormones, the ebb phase represents a distributive shock characterised by arterial vasodilatation and transcapillary albumin leak abating plasma oncotic pressure. Arterial underfilling, microcirculatory dysfunction and secondary interstitial oedema lead to systemic hypoperfusion and regionally impaired tissue use of oxygen. In this early stage of shock, adequate fluid therapy comprises adequate goal directed filling to prevent evolution to multiple organ dysfunction syndrome (MODS). As compensatory neuroendocrine reflexes and potential renal dysfunction could result in sodium and water retention, positive fluid balances are inherent to the ebb phase. Patients with a higher severity of illness need more fluids to reach cardiovascular optimisation; therefore, at this point fluid balance may be considered a biomarker of critical illness (Bagshaw and Bellomo 2007). Patients overcoming shock typically attain homeostasis of proinflammatory and anti-inflammatory mediators within three days. Subsequent haemodynamic stabilisation and restoration of plasma oncotic pressure set off the flow phase with resumption of diuresis and mobilisation of extravascular fluid, resulting in negative fluid balances.
The Three Hit Model of Shock
Recent studies have shown that the application of conservative late fluid management (CLFM), with two consecutive days of negative fluid balance within the first week of stay, offers a strong chance of survival (Murphy et al. 2009). In contrast, patients with persistent systemic inflammation maintain transcapillary albumin leak and do not reach the flow phase, mounting up positive fluid balances. In this context, the global increased permeability syndrome (GIPS) has been introduced, characterised by a high capillary leak index (CLI, expressed as CRP over albumin ratio), excess interstitial fluid, persistent high extravascular lung water index (EVLWI), no CLFM achievement and progressing organ failure (Cordemans et al. 2012). GIPS represents a third hit, following the acute injury (as first hit) with progression to MODS (as the second hit) (Malbrain and De Laet 2008). The third hit may develop in patients that do not enter the flow phase spontaneously. Figure 1 summarises this three hit model.
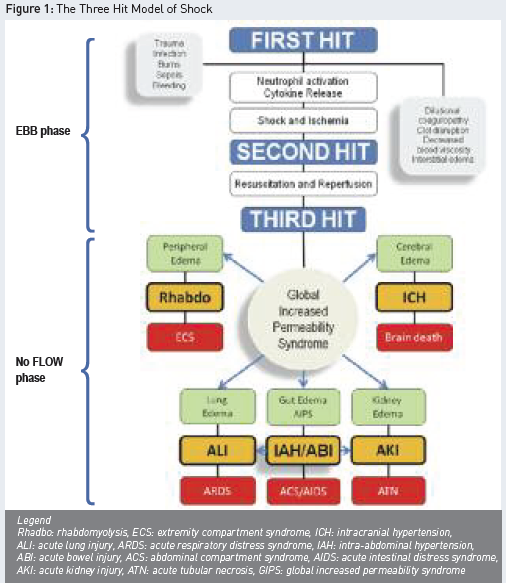
The dual response to acute inflammatory insult is characterised by a crucial turning point on day three. Presumably, homeostasis of cytokines on the third day after shock onset allows initiation of healing the microcirculatory disruptions and closure of capillary leak. This interpretation is supported by observations demonstrating normalisation of microcirculatory blood flow on day three in patients with abdominal sepsis. Lower EVLWI and pulmonary vascular permeability indices on day three of shock have been shown to correlate with better survival (Kuzkov et al. 2006).
Polycompartment Syndrome
In cases of capillary leak and the impaired flow stage, overzealous administration of fluids in the GIPS phase will lead to gross fluid overload and tissue oedema. Interstitial oedema raises the pressure in all four major body compartments: head, chest, abdomen and extremities, and as a result, venous resistance of organs within compartments increases and perfusion pressure decreases, contributing to the progression of organ failure. As different compartments interact and reciprocally transmit compartment pressures, polycompartment syndrome is suggested to occur (Malbrain and Wilmer 2007; Malbrain and De Laet 2008 and 2009).
The abdomen plays a central role in GIPS and polycompartment syndrome, as positive fluid balances are a known risk factor for secondary intra-abdominal hypertension (IAH), which is in turn associated with adverse effects on other compartments and organ functions (Malbrain et al. 2006).
Pathophysiology
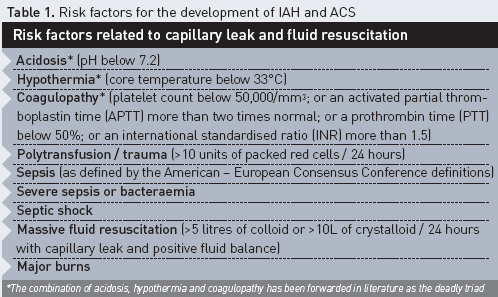
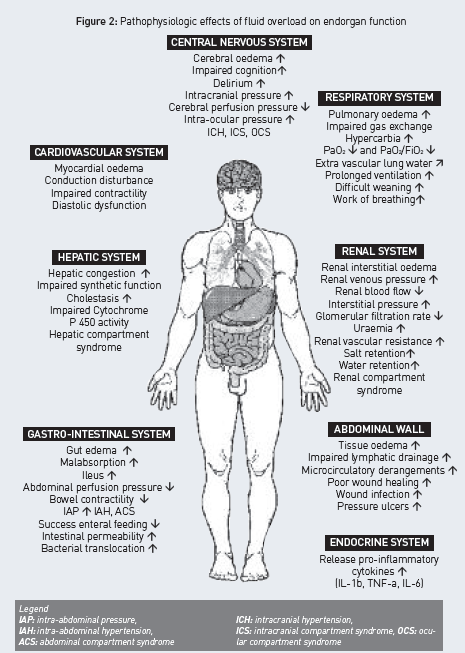
Renal function in particular is strongly affected by IAH. Furthermore, renal interstitial oedema in absence of IAH may impair renal function. Therefore, fluid overload leading to IAH and associated renal dysfunction may counteract its own resolution (Table 1) (De Laet et al. 2007). The adverse effects of fluid overload and interstitial oedema are numerous and have an impact on all endorgan functions, as illustrated in Figure 2, although some colleagues recently suggested that peripheral oedema is only of cosmetic concern (Pinsky 2007).
When adverse effects of fluid overload in states of capillary leak are particularly pronounced in the lungs, monitoring of EVLWI may be a valuable tool in the guidance of fluid management in the critically ill. A high EVLWI indicates a state of capillary leak associated with a higher severity of illness and mortality, and recent studies have correlated EVLWI with albumin extravasation in patients after multiple trauma.
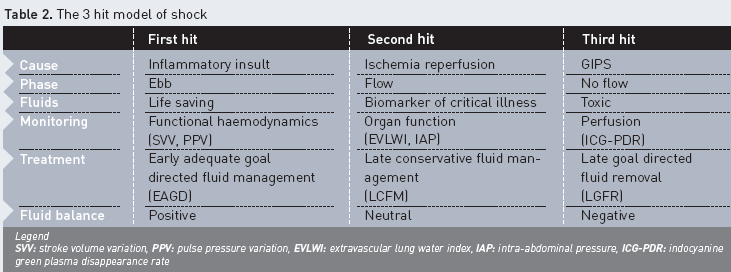
Responders to CLFM overcome the distributive shock and make a transition to the flow phase; however, non-responders stay in the grip of the ebb phase and progress to GIPS, resulting in positive fluid balances, organ failure and death. In this hypothesis, (change in) EVLWI has a prognostic value as a reflection of the extent of capillary leak, rather than as a quantification of lung function impairment by lung water (Cordemans et al. 2012). These recent observations are important to bear in mind when making decisions on fluid management in critically ill patients with IAH. Patients at risk for GIPS, as assessed by CLI, IAP, changes in EVLWI and fluid balance, require restrictive fluid strategies and even fluid removal guided by extended haemodynamic monitoring, including lung water measurements (late goal directed fluid removal). Previously, the application of EVLWI-guided fluid therapy led to improved outcomes and lower positive fluid balances in states of capillary leak (Mitchell et al. 1992). Restrictive fluid management may necessitate a greater use of vasopressor therapy, resuscitation with hyperoncotic solutions (e.g. albumin 20%), and early initiation of diuretics and renal replacement therapy. Within the concept of dual response to shock, it is possible to identify patients with persistent capillary leak that do not reach the flow phase. In this context, GIPS reflects a third hit of shock, which arises after acute injury and MODS. In those patients, superfluous fluid administration results in oedema formation, progression of organ failure and worse outcomes, and may be considered toxic (Table 2). Therefore, as soon as haemodynamics allow, early transition to conservative fluid management and even fluid removal on the basis of EVLWI-guided protocol is mandated (late goal directed fluid removal) (Malbrain and De Laet 2008; Murphy et al. 2009; Cordemans et al. 2012; Cordemans et al. 2012).
Key Message
Capillary leak is an inflammatory condition with diverse triggers that results from a common pathway, including ischemia-reperfusion, toxic oxygen metabolite generation, and cell wall and enzyme injury, leading to a loss of capillary endothelial barrier function. In this circumstance, plasma volume expansion to correct hypoperfusion predictably results in extravascular movement of water, electrolytes and proteins. Peripheral tissue oedema, visceral oedema and ascites may be anticipated in proportion to the volume of prescribed resuscitation fluid; thus, a variety of strategies are available to the clinician to reduce the volume of crystalloid resuscitation utilised while restoring macro- and microcirculatory flow. Regardless of the resuscitation strategy, the clinician must maintain a heightened awareness of the dynamic relationship between capillary leak, fluid loading, peripheral oedema, intraabdominal hypertension and abdominal compartment syndrome.
References:
For references please send a request to





