ICU Management & Practice, Volume 22 - Issue 3, 2022
A million surgical procedures performed every year worldwide are at risk of complications that can be attributed to the nature of the surgery and/or the physiological status of the patient. Although high-risk surgical patients represent only 10-15% of surgical procedures, they account for more than 80% of deaths and might benefit from perioperative haemodynamic monitoring, which refers to the haemodynamic optimisation of fluids, vasopressors, and inotropes to predefined physiological targets to maintain or restore sufficient oxygen delivery to the tissues. In fact, perioperative haemodynamic optimisation therapy has a significant impact on the outcomes of high-risk patients undergoing major surgery, potentially decreasing the length of ICU and hospital stay, morbidity and mortality (Fellahi et al. 2021).
Historic Background
The oxygen debt was first hypothesised in 1922 (Hill and Lupton 1922; Hill and Lupton 1923). They theorised that the body needs to replace the oxygen used by working muscles during mild to intense exercise. Decades later, Shoemaker et al. (1992) demonstrated the role of oxygen debt in the development of organ failure and death in high-risk surgical patients. In 1985, Schultz et al. (1985) demonstrated the benefit of physiologic monitoring with pulmonary artery catheters in patients with fractures of the hip. In 1988, Shoemaker et al. (1988) demonstrated that targeting specific values for the cardiac index, oxygen delivery and oxygen consumption using fluids and inotropes to achieve these goals resulted in a reduction in mortality and morbidity. Since then, there have been several randomised controlled trials and meta-analyses supporting the practice of perioperative haemodynamic optimisation (PHO) (Cove et al. 2012).
Physiological Concepts
Oxygen delivery (DO2) is determined by central and peripheral mechanisms. Among the central factors, DO2 is determined by the product of cardiac output (CO) and arterial oxygen content (CaO2). CaO2 is defined as the amount of oxygen bound to haemoglobin (Hb) plus the oxygen dissolved in plasma. Changes in Hb concentration and arterial oxygen saturation (SaO2) can be compensated by an increase in CO. However, the converse is not true, as the arterial content depends on CO to reach tissues. A basic example is blood transfusion. It would be logical to expect an elevation in Hb to systematically and predictably elevate DO2. However, this is not what is observed, since, in addition to generating an inflammatory response, which impairs the microcirculation, the increase in viscosity can lead to a reduction in CO. Thus, the importance of haemodynamic monitoring that provides information about CO and the adequacy of perfusion and oxygenation of the tissues and organ systems. In addition, peripheral mechanisms should also be considered because they can be altered in inflammatory conditions, disfiguring the control of vascular tonus and providing the formation of microthrombi, which obstruct capillary circulation and lead to an irregular distribution of blood flow.
In the surgical context, the increase in cellular oxygen demand due to the metabolism acceleration is relevant. Major surgical trauma elevates the mean oxygen demand from 110 mL/min/m2 at rest to 170 mL/min/m2. In most patients, this increase in demand is accompanied by an increase in CO and tissue oxygen extraction. Nevertheless, patients with little functional reserve may be unable to increase their CO under conditions that accompany the increase in tissue demand, generating hypoxia, cell death, and multiple organ failure. Briefly, on the one hand, increased oxygen demand generated by tissue injury, the endocrine-metabolic response, and other factors such as stress and hyperthermia. On the other hand, comorbidities prevent an adequate increase in oxygen delivery through the increase in CO. The use of inotropic, fluids and vasoactive drugs can increase oxygen supply and may reduce the imbalance between supply and demand of oxygen reducing complications.
Importance of Perioperative Haemodynamic Monitoring
The risk of perioperative complications is related to the patient's condition and comorbidities, the type of surgery performed and its duration, the degree of urgency, the skills and experience of the surgical and anaesthetic teams, and the postoperative management. Insufficient tissue perfusion and cell oxygenation due to hypovolaemia and/or cardiac dysfunction are major causes of perioperative complications and unfavourable outcomes. Low cardiorespiratory reserve seems to be the key factor in the aetiology of complications, which explains its higher incidence in elderly patients with comorbidities and with low functional reserve. Therefore, maintaining adequate DO2 for cells is critical.
Is There Really Evidence of Benefit?
Three important reviews demonstrated that PHO leads to a reduction in perioperative mortality, potentially by reducing the number of postoperative complications. A meta-analysis of 29 PHO studies (Hamilton et al. 2011) found a reduction in morbidity (OR 0.43; 95% CI 0.35-0.55) and mortality (OR 0.48; 95% CI 0.33-0.70) in patients undergoing PHO but noted that the subgroup analysis showed that the mortality benefit was predominant in older studies using pulmonary artery catheter (PAC), fluid-associated inotropic drugs, and those whose haemodynamic goals were aimed at supranormal values. In a systematic review of 32 studies (5056 high-risk surgical patients) of PHO aiming at maintaining tissue perfusion, the authors (Gurgel et al. 2011) found that although PHO reduced the incidence of organ dysfunction in all patients, mortality was only reduced in the cohort of patients with baseline mortality greater than 20% in the control group (OR 0.67; 95% CI 0.55-0.82). A large multicentre, prospective, randomised study of perioperative optimisation versus usual care in high-risk patients undergoing major gastrointestinal surgery showed no difference in postoperative morbidity and mortality, although an updated meta-analysis of these same data showed a reduction in morbidity (RR 0.77, 95% CI 0.71-0.83) with the use of PHO (Pearse et al. 2014).
It is worth highlighting the importance of postoperative complications in major surgeries as predictors of long-term survival. Rhodes et al. (2010) evaluated the long-term survival of patients included in previous PHO RCTs for high-risk surgical patients. They found that 15 years after the original study, long-term survival was related to the PHO group and avoidance of cardiovascular complications. Therefore, the benefits conferred by PHO seem to be linked to several characteristics that consistently appear in these studies:
- use of CO monitors
- use of protocols defined by the clinical team
- early onset of PHO
What is the Real Importance?
Patients surviving major surgery are those with the ability to increase their DO2 and VO2 to supranormal values (Bishop et al. 1993). Monitoring CO and DO2 has now become standard clinical practice to provide adequate tissue oxygenation and forms the basis of PHO. There are currently many CO monitors available for clinical use, with different degrees of invasiveness and measured variables. Below are some of the techniques of haemodynamic monitoring devices most used in clinical practice:
- Pulmonary artery catheter (PAC)
- Blood pressure waveform analysis
- Doppler technique for CO monitoring
- O2 central venous saturation (ScvO2), O2 extraction rate (O2ER) and lactate
Faced with so many alternatives, the choice of the monitoring device must be made based on the following factors (Alhashemi et al. 2011):
- institutional (availability, level of experience, compatibility with existing monitors)
- related to the device (invasiveness, technical limitations, validation and accuracy)
- Patient-specific (arrhythmias, contraindications for insertion, type of surgery and type of treatment protocol)
How to Choose Which Goal to Use and Conduct the Protocol?
For the performance of PHO therapy, specific physiological data of each patient are used to guide interventions that enable the achievement of adequate tissue blood flow goals. Unfortunately, to date, there is no ideal goal for PHO. An ideal goal should:
- • reflect organic perfusion
- • be readily available in the perioperative period
- • generate continuous measures
- • be easily reproducible
The ultimate goal of this strategy is to prevent cellular dysoxia through an adequate relationship between DO2 and VO2. As VO2 depends on each patient's own factors, we can actively work to optimise DO2, which is governed by the following equation:
DO2 = (SaO2 x Hb x 1.34) x (SV x HR)
The first step is to maximise the systolic volume (SV) through the infusion of intravenous (IV) fluids titrated according to the haemodynamic response. The goal is to achieve a preload that contributes to an "almost maximum" SV or CO in accordance with Frank-Starling laws. This is technically known as a “proof” or “challenge” of volume. Thus, the clinician can administer fluid, and at the same time, test the patient's recruitable preload reserve (Cecconi et al. 2011). If the target (e.g., DO2) has not yet been reached and the patient is already in the “plateau” region of the Frank-Starling curve (not responsive to IV fluids), inotropics can be introduced to improve the SV/CO and accordingly DO2. In terms of mortality reduction, the combination of fluids and inotropics was superior to only fluids (Lobo 2006; Hamilton et al. 2011).
The basic model for a perioperative optimisation protocol can be seen in the organogram in Figure 1. Several other indices related to blood flow, tissue perfusion or fluid responsiveness, in addition to DO2, have been used in recent years, in general: flow time corrected (FTc), that is the flow time duration of blood flow in the aorta in oesophageal Doppler monitoring (Abbas 2008), venous oxygen saturation (SvO2) (Collaborative Study 2006), oxygen extraction rate (O2ER) (Donati et al. 2007), lactate concentration (Polonen et al. 2000), and pulse pressure variation (PPV) (Malbouisson et al. 2017). Nonetheless, apparently the use of DO2 and cardiac index (CI) as endpoints conferred mortality reduction. It is noteworthy that the effects on mortality were more evident when used supranormal values of DO2 as the resuscitation goal (Poeze et al. 2005; Hamilton et al. 2011; Brienza et al. 2009). However, for the prevention of complications, normal goals seem to be as effective as supernormal goals (Brienza et al. 2009; Rhodes et al. 2010). Evidence also suggests that the use of protocols in PHO is associated with better results.
When to Start PHO?
The replacement of oxygen debt is therefore time sensitive, and once the cell and mitochondrial structure are permanently damaged, attempts to improve oxygen flow are futile (Abid et al. 2000). Oxygen debt can be repaired in the early phases of the systemic inflammatory reaction (SIRS) that accompanies surgery through flow optimisation of oxygen to tissues.
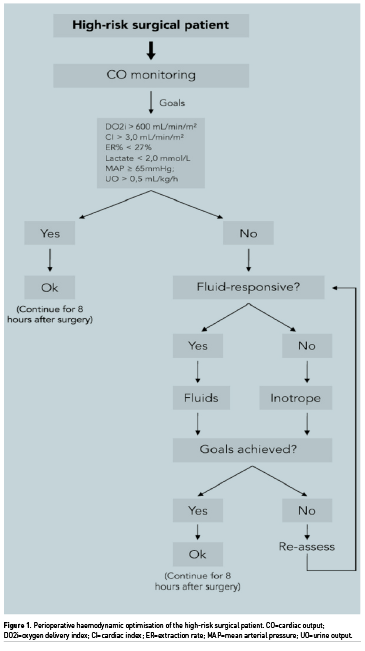
Early optimisation of oxygen flow goals in high-risk surgical patients before the development of organ failure was associated with a significant reduction in mortality (Kern et al. 2002). Patients with an optimisation strategy initiated after the development of organ failure do not present an improvement in mortality. A systematic review and meta-analysis of 26 RCTs was performed with patients undergoing major surgery, where only studies that started haemodynamic optimisation early (up to 8 h from the start of surgery) were included. Similar results to two previous meta-analyses found that pre-emptively performed GDT was associated with reduced postoperative ARF and gastrointestinal complications (Brienza et al. 2009; Giglio et al. 2009).
.jpg)
Economic Impact of PHO
Regardless of the location or the costing modality, the use of haemodynamic handling packages has costs, which may be inherent to the tool itself and/or to the set of interventions performed. However, studies indicate good cost-effectiveness of PHO (Fenwick et al. 2002; Barthaet al. 2012). A meta-analysis (Silva-Jr et al. 2020) from the perspective of a developing country demonstrated a significant total cost reduction in the group of surgical patients who underwent monitoring interventions, with savings of $90,161 USD for every 1000/patients/treated, and a shorter ICU and hospital length of stay compared with the control group.
Conclusion
A significant body of evidence supports perioperative haemodynamic optimisation in high-risk surgery patients. The basic principle of optimisation is based on manipulation of oxygen delivery to improve patient outcome by targeted administration of intravenous fluids, vasopressors, inotropes and blood. CO monitoring is an important component of perioperative haemodynamic optimisation. Preload, afterload, and contractility can be evaluated with a number of haemodynamic monitoring tools that are validated but differ in invasiveness, technology, advantages, and limitations. A profound understanding of the different CO monitoring methods is essential in defining which method will be used (Michard et al. 2019; Lobo and Oliveira 2013; Lobo et al. 2013). Strategies should be developed to facilitate the implementation and adoption of perioperative haemodynamic monitoring in clinical practice to improve patient outcomes.
Conflict of Interest
None.
References:
Abbas SM, Hill AG (2008) Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 63: 44-51.
Abid O, Akca S, Haji-Michael P, Vincent JL (2000) Strong vasopressor support may be futile in the intensive care unit patient with multiple organ failure. Crit Care Med. 28:947–949.
Alhashemi JA, Cecconi M, Hofer CK (2011) Cardiac output monitoring: an integrative perspective. Crit Care. 15: 214.
Bartha E, Davidson T, Hommel A et al. (2012) Cost-effectiveness analysis of goal-directed hemodynamic treatment of elderly hip fracture patients: before clinical research starts. Anesthesiology. 117(3):519–30.
Bishop MH, Shoemaker WC, Appel PL et al. (1993) Relationship between supranormal circulatory values, time delays, and outcome in severely traumatized patients. Crit Care Med. 21: 56–63.
Brienza N, Giglio MT, Marucci M, Fiore T (2009) Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 37: 2079–2090.
Cecconi M, Parsons AK, Rhodes A (2011) What is a fluid challenge? Curr Opin Crit Care. 17: 290–295.
Collaborative Study Group on Perioperative ScvO2 Monitoring (2006) Multicentre study on peri- and postoperative central venous oxygen saturation in high-risk surgical patients. Crit Care. 10: R158.
Cove ME, Pinsky MR (2012) Perioperative hemodynamic monitoring. Best Pract Res Clin Anaesthesiol. 26(4):453-62.
Donati A, Loggi S, Preiser JC et al. (2007) Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 132:1817-1824.
Fellahi JL, Futier E, Vaisse C et al. (2021) Perioperative hemodynamic optimization: from guidelines to implementation-an experts' opinion paper. Ann Intensive Care. 11(1):58.
Fenwick E, Wilson J, Sculpher M, Claxton K (2002) Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive care med. 28(5):599-608.
Giglio MT, Marucci M, Testini M, Brienza N (2009) Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 103: 637–646.
Gurgel ST, do Nascimento P Jr (2011) Maintaining tissue perfusion in high-risk surgical patients: a systematic review of randomized clinical trials. Anesth Analg. 112:1384-1391.
Hamilton MA, Cecconi M, Rhodes A (2011) A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 112:1392-1402.
Hill AV, Lupton H (1922) The oxygen consumption during running. J. Physiol. 56(xxxii).
Hill AV, Lupton H (1923) Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med. 16:135-71.
Kern JW, Shoemaker WC (2002) Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 30: 1686-1692.
Lobo SM, Lobo FR, Polachini CA et al. (2006) Prospective, randomized trial comparing fluids and dobutamine optimization of oxygen delivery in high-risk surgical patients [ISRCTN42445141]. Crit Care. 10(3):R72.
Lobo SM, de Oliveira NE (2013) Clinical review: What are the best hemodynamic targets for noncardiac surgical patients? Crit Care. 17(2):210.
Lobo SM, Mendes CL, Rezende E, Dias FS (2013) Optimizing perioperative hemodynamics: what is new? Curr Opin Crit Care. 19(4):346-52.
Malbouisson LMS, Silva JM, Carmona MJC et al. (2017) A pragmatic multi-center trial of goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery. BMC Anesthesiol.17:70.
Michard F, Biais M, Lobo SM, Futier E (2019) Perioperative hemodynamic management 4.0. Best Pract Res Clin Anaesthesiol. 33(2):247-255.
Pearse RM, Harrison DA, MacDonald N et al. (2014) Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 311:2181-2190.
Poeze M, Greve JW, Ramsay G (2005) Meta-analysis of hemodynamic optimization: relationship to methodological quality. Crit Care. 9: R771-R779.
Polonen P, Ruokonen E, Hippelainen M et al. (2000) A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 90:1052–1059.
Rhodes A, Cecconi M, Hamilton M et al. (2010) Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med. 36:1327-1332.
Schultz RJ, Whitfield GF, LaMura JJ et al. (1985) The role of physiologic monitoring in patients with fractures of the hip. J Trauma. 25(4):309-16.
Shoemaker WC, Appel PL, Kram HB et al. (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest.
Shoemaker WC, Appel PL, Kram HB et al. (1992) Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 102(1):208-15.
Silva-Jr JM, Menezes PFL, Lobo SM et al. (2020) Impact of perioperative hemodynamic optimization therapies in surgical patients: economic study and meta-analysis. BMC Anesthesiol.20:71.











