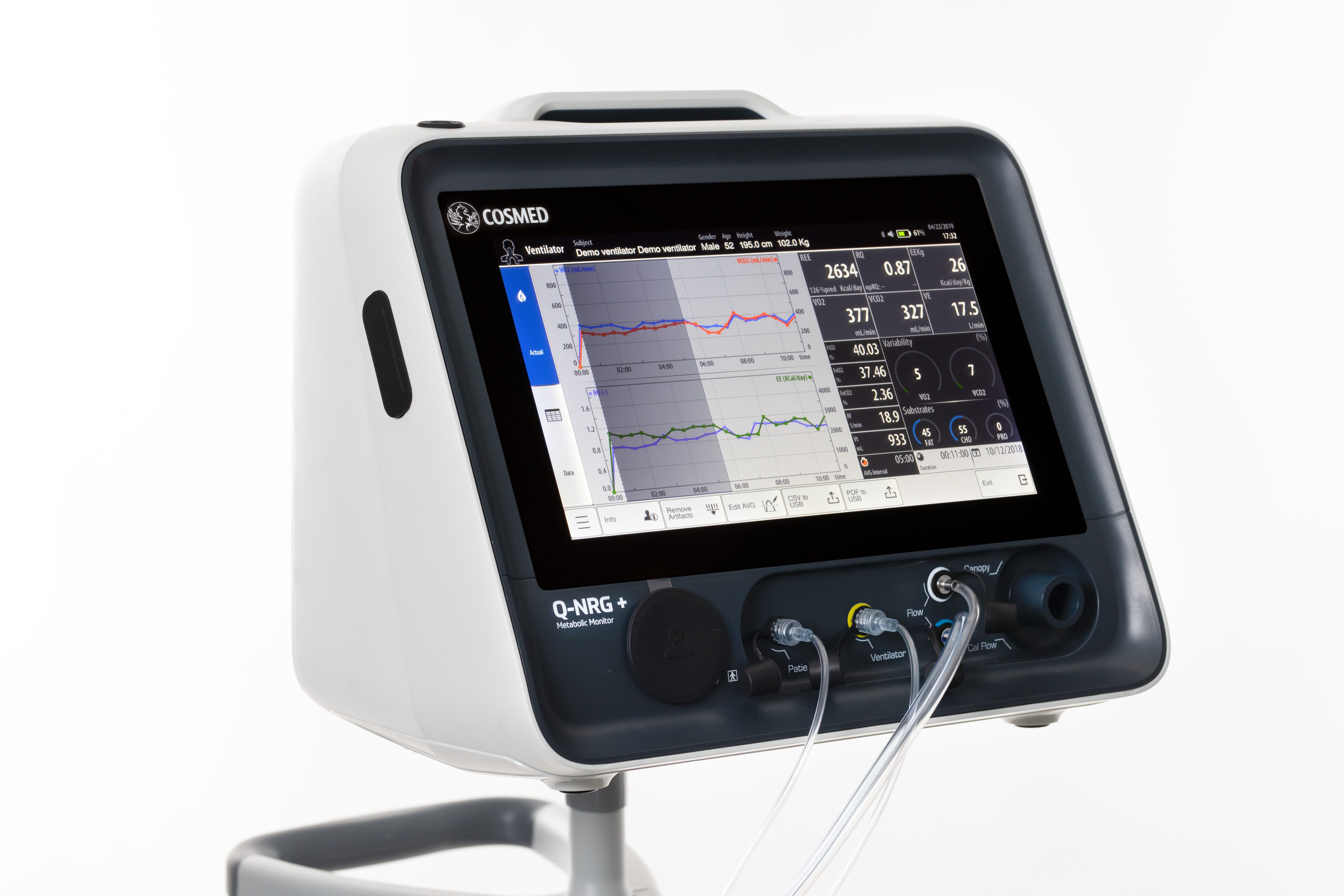
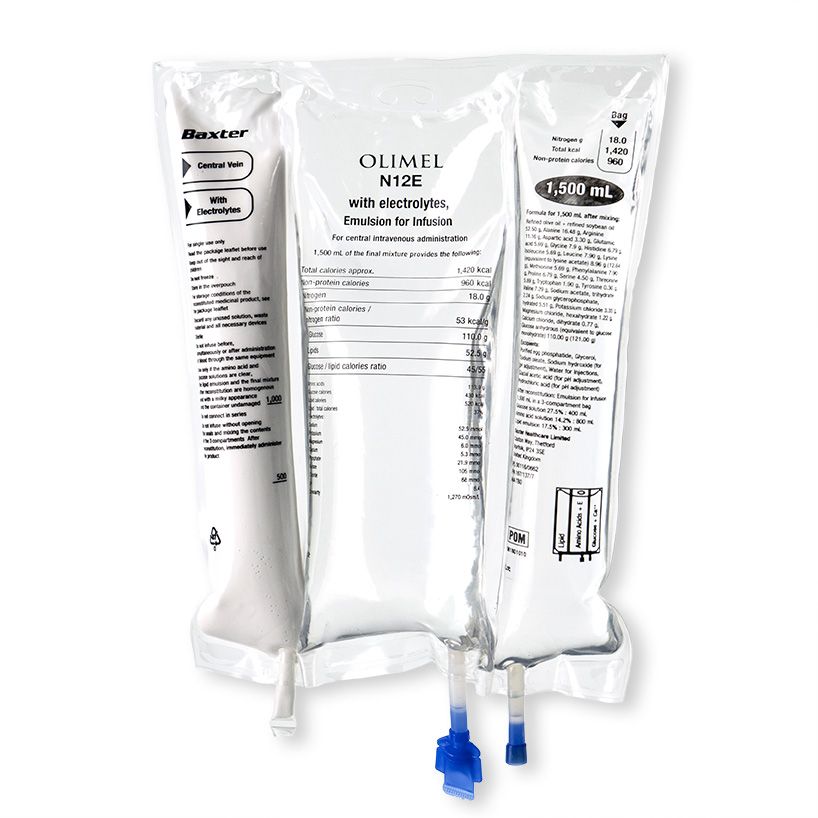
Baxter launches a new parenteral nutrition formulation designed to meet the need for higher protein provision in Europe and signs a global partnership with COSMED to commercialise Q-NRG+, a metabolic monitoring device that utilises indirect calorimetry technology.
Malnutrition in the ICU
Around 20 to 50% of hospital patients, including those in the ICU (trauma, surgery) are malnourished.1 Malnutrition is a clinical condition that affects multiple patient groups and can have a significant impact on both clinical outcomes and healthcare systems, as it is typically associated with higher infection rates, increased morbidity and mortality, longer hospital stays, increased healthcare costs and reduced quality of life.
Critical illness often results in rapid protein breakdown and muscle loss. Clinical evidence shows that nutrition that involves both moderate energy intake and high protein is associated with reduced mortality. Both the European Society of Nutrition and Metabolism (ESPEN) and the American Society for Parenteral and Enteral Nutrition (ASPEN) recommend a higher protein intake with controlled calorie for critically ill patients.2 3
Clinical Nutrition Innovations
Clinical nutrition in the ICU has evolved over the years. Baxter International Inc., a global leader in clinical nutrition, has been a part of this journey, listening to clinicians and addressing the unmet needs in the critical care segment in line with nutritional guidelines that have moved towards acknowledging the importance of meeting protein targets in critically ill patients. Baxter have recently launched a number of innovations that will enable clinicians to identify and address malnutrition in the critically ill. Along with COSMED, a worldwide leader in the design of metabolic systems for clinical and human performance application, Baxter will be commercialising Q-NRG+; a metabolic monitoring device that utilises indirect calorimetry technology, in 18 key countries around the world with potential for future expansion.
Indirect calorimetry (IC) is the gold standard4 for measuring resting energy expenditure (REE) - a patient’s calorie needs while at rest. The ESPEN guidelines recommend that in critically ill mechanically ventilated patients, energy needs should be determined using indirect calorimetry.2 The ASPEN guidelines also suggest that IC be used to determine energy requirements, and in the absence of IC, a predictive equation or a weight- based equation be used.3
Q-NRG+ represents the next generation of IC technology and enables individualised monitoring measurements to help clinicians optimise nutrition therapy in critically ill patients. Q-NRG+ is designed to address barriers to rapidly and accurately measure a patient's REE.5 It is flexible, portable, and easy to use by all clinicians. The device requires minimal warm-up and calibration time and can deliver readings in as few as five minutes.
Q-NRG+ is a unique product that is a result of collaboration with world- class institutes in the eld of nutrition support in ICUs. It is simple to use and can solve typical pitfalls of previous IC technology. With Q-NRG+, clinicians will now have access to an effective and practical tool that can be used at the point of care.
Baxter have also launched in Europe, their latest addition to their olive oil- based parenteral nutrition (PN) triple chamber bag portfolio; Olimel N12. The new PN bag combines a high protein formulation with low glucose content, resulting in the lowest energy to protein ratio currently available in a standardised, triple-chamber bag. The new formulation contains 76g of amino acid per liter (designed to meet protein targets in lower fluid volumes) and only 73g of glucose per liter (helping to reduce the potential glycaemic load and subsequent risk of hyperglycaemia). The olive-oil based lipid emulsion may preserve immune function.6 7 8 9 10
Future Outlook
As clinical nutrition continues to gain importance in the ICU setting, the need for advanced strategies and tools that could help clinicians ensure patients receive adequate nutrition for better long-term outcomes and improved quality of life will be fundamental. Baxter is committed in its role as a leader in clinical nutrition and continues to partner with institutions and clinicians to develop innovations that will improve outcomes for critically ill patients.
* Important Safety/Risk InformatIon for Olimel N12
Therapeutic indications:
PERIOLIMEL/OLIMEL are indicated for parenteral nutrition for adults and children greater than 2 years of age when oral or enteral nutrition is impossible, insufficient or contraindicated.
PERIOLIMEL/OLIMEL are not recommended for use in children less than 2 years of age due to inadequate composition and volume.
Contraindications:
The use of PERIOLIMEL/OLIMEL with and without electrolytes are contra-indicated in the following situations:
- In premature neonates, infants and children less than 2 years of age
- Hypersensitivity to egg, soybean, or peanut proteins, or to any of the active substances or excipients
- Congenital abnormalities of amino acid metabolism
- Severe hyperlipidaemia or severe disorders of lipid metabolism characterised by hypertriglyceridaemia
- Severe hyperglycaemia
The use of peRIoLImeL/oLImeL with electrolytes are contra-indicated in the following situations:
- Pathologically-elevated plasma concentrations of sodium, potas- sium, magnesium, calcium, and/or phosphorus.
Baxter and Olimel are registered trademarks of Baxter International Inc
Q-NRG is a registered trademark of COSMED