ICU Management & Practice, ICU Volume 15 - Issue 3 - 2015
Epidemiology, Pathophysiology, Diagnostics and Treatment
Due to malnutrition and aspiration dysphagia in critically ill patients on the ICU is an extremely important symptom with crucial impact on outcome and mortality. A broad variety of pathogenetic factors can lead to severe dysphagia in non-intubated and intubated patients followed by a significant delay in decannulation after weaning from the respirator has been completed. The aim of the following review is to present the diversity of pathogenetic factors on the ICU, evaluate the existing diagnostic procedures and, based on current knowledge, give pragmatic recommendations for the diagnostic approach as well as for the further nutritional management of ICU patients.
In intensive care medicine dysphagia is an extremely frequent and outcome-relevant symptom. Studies on internal medicine, anaesthesiological and surgical intensive care units have shown that 50 to 70% of patients on these wards suffered from dysphagia (Ajemian et al. 2001; Skoretz et al. 2010). In a recent study on a neurological intensive care unit there was even a dysphagia incidence of over 90%, and dysphagia persisted in half of the patients until the day of discharge (Macht et al. 2013). Of particular relevance is the finding that dysphagia in intensive care patients is more severe and in 10-20% of the patients accompanied by silent aspirations (Ajemian et al. 2001, Barquist et al. 2001, El Solh et al. 2003). Regardless of the diagnostic spectrum analysed, dysphagia in critically ill patients is a significant predictor of complications, especially aspiration pneumonia and reintubation, and a crucial determinant of the duration of hospitalisation and the patients’ outcome (Macht et al. 2011).
Aetiology and Pathophysiology of Dysphagia on the ICU
The causes of dysphagia in the critically ill can be differentiated into three aetiological categories. Thus, dysphagia may be:
(i) associated with the main diagnosis leading to ICU treatment;
(ii) The result of co-morbidities;
(iii) Associated with the treatment on the ICU itself.
Especially in patients on the neurological intensive care unit a combination of the different aetiologies must be expected.
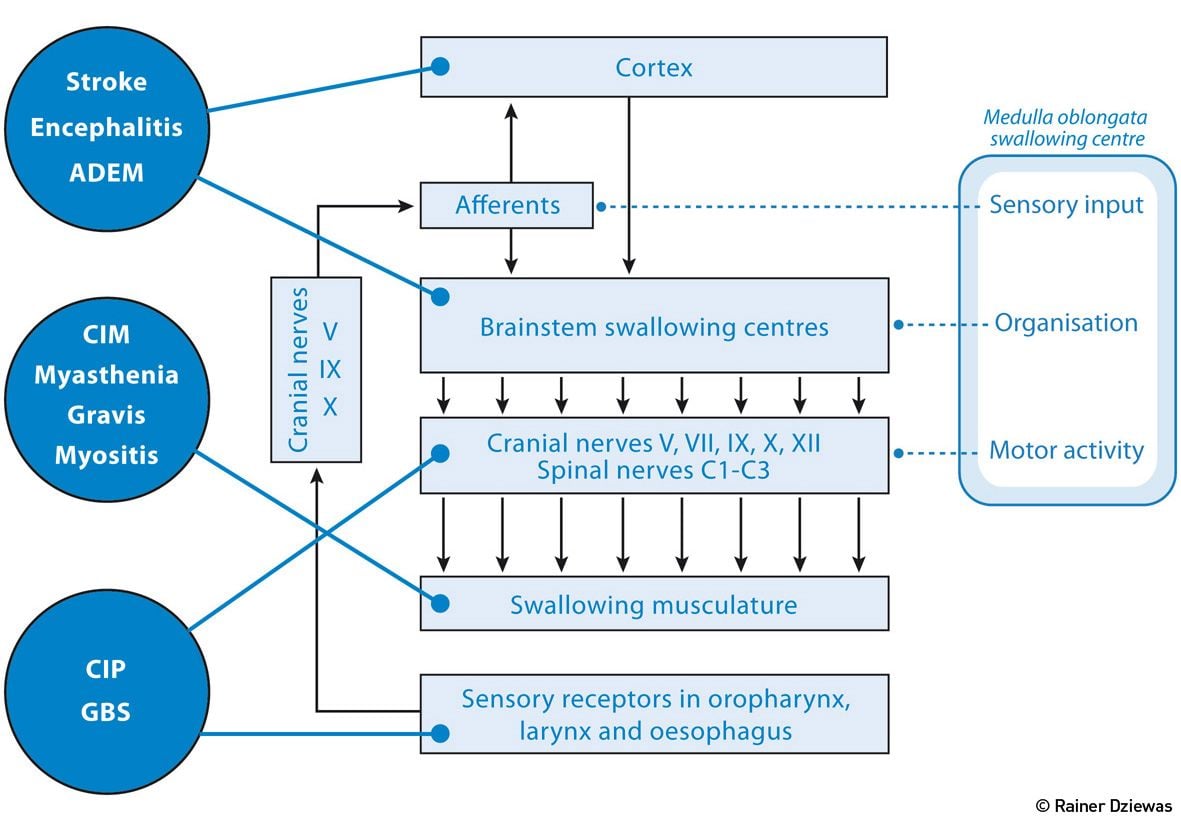
Figure 1.Neurological Disorders that Typically Require Treatment in the ICU
(i) Diagnosis-Associated
As shown in Figure 1, various neurological disorders that typically require treatment on the ICU impair the functionality of the swallowing network or the associated downstream nerves and muscles. Stroke and inflammatory diseases of the CNS lead, depending on location, to disturbance of the supramedullary or medullary control of swallowing. Guillain-Barré syndrome (GBS), critical illness neuropathy (CIP) and critical illness myopathy (CIM) cause dysphagia due to an impairment of motor and sensory cranial nerve function. Finally, disorders of swallowing muscles themselves, as they can be observed in inflammatory myositis, as well as disorders involving the neuromuscular junction, lead to myogenic dysphagia.
(ii) Caused by Co-Morbidities
Apart from the main diagnosis (e.g. acute stroke, GBS, brainstem encephalitis), co-morbidities also play an important role. A wide range of neurodegenerative (e.g., Parkinson's disease, Alzheimer's disease), neurovascular (stroke, subcortical arteriosclerosis encephalopathy) or neuromuscular disorders (polymyositis, ALS) should be mentioned. These disorders are either associated with pre-existing dysphagia, or at least increase the likelihood of a deterioration of swallowing function during ICU treatment.Thus, although hospitalisation on the ICU may be initiated because of e.g. urosepsis or myocardial infarction, the further clinical course gets complicated because of decompensated dysphagia.
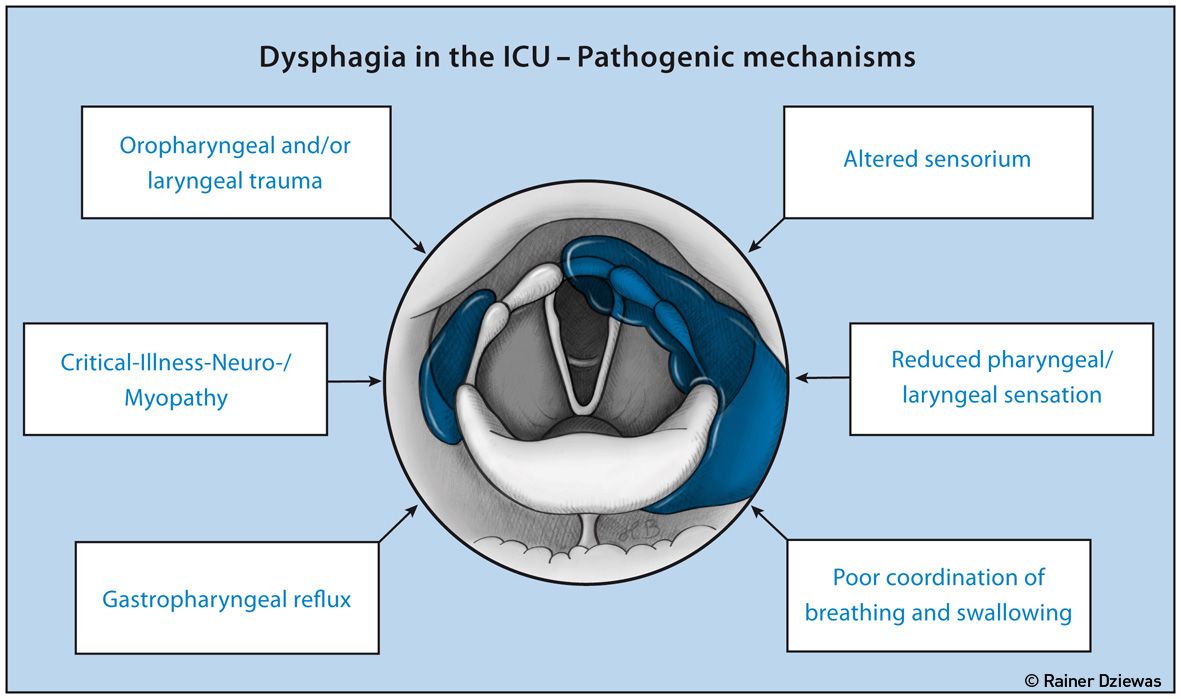
Figure 2. Pathomechanisms of ICU-Related Dysphagia
(iii) ICU Treatment-Related
Dysphagia in the ICU may also be caused by the treatment itself and/or further environmental conditions. There are six athomechanisms to differentiate as shown in Figure 2 (Macht et al. 2013).
1. The endotracheal tube, the tracheal cannula, laryngeal masks and nasogastric suction probes can lead to various injuries of the pharynx, larynx or oesophagus.
2. Intensive care patients often develop a weakness of the swallowing muscles due to critical illness neuropathy and myopathy.
3. The development of oropharyngeal and laryngeal sensory deficits. Among other reasons this may be the result of sensory nerve damage due to CIP or because of local mucosal oedema followed by a disruption of the sensory feedback.
4. Qualitative and quantitative impairment of consciousness, either as an effect of sedating medication or as a result of delirium, are also involved in the development of dysphagia.
5. Gastroesophageal reflux in critically ill patients causes insufficient supply of nutrients and is in particular a main risk factor for aspiration.
6. Patients on the ICU often suffer from a desynchronisation of breathing and swallowing. Both the duration of the swallowing apnoea, as well as the coordination of the respiratory cycle and the moment of swallowing may be impaired, increasing the risk of aspiration (Shaker et al. 1992, Gross et al. 2009).
Diagnostic Workup
Dysphagia plays an important role on the ICU. Adequate diagnostic procedures should help to detect this disorder as precisely and timely as possible. Based on the result of swallowing assessment appropriate nutritional management and treatment strategies have to be defined. This section describes a workflow applicable to the ICU.
Screening Tests for Aspiration
The aim of dysphagia screening on the ICU is to identify patients at risk of aspiration, and subsequently to initiate preventive measures and to plan further diagnostic procedures. To this end, water swallowing tests are usually implemented. As a common feature of several different published protocols, the patient is asked to swallow a defined amount of water, while the investigator looks for clinical signs of aspiration (change in voice, cough, stridor) (Cassier-Woidasky et al.2012). However, these tests usually do not have sufficient sensitivity and/or specificity (Bours et al. 2009) to be propagated as a stand-alonesolution. In addition silent aspiration, a key factor in the critically ill, cannot be detected by these tests (Noordally et al. 2011). Finally it should be noted that in many critically ill patients a water test is not feasible due to their clinical condition, so that in the end both the validity and the feasibility of these water tests in the ICU are significantly limited.
Clinical Examination
The clinical swallowing examination by an appropriately trained speech therapist is the most frequently used diagnostic modality for the evaluation of dysphagia on the ICU. This typically involves examination of the oropharyngeal structures as well as swallowing tests with different consistencies (Warnecke and Dziewas 2013). As with aspiration screening, the sensitivity, specificity and reliability of the clinical swallowing examination are also questionable (McCullough et al. 2000; 2001). Hales et.al. (2008) found in a prospective study of 25 tracheotomised ICU patients a sensitivity of only 66% for the detection of aspirations with a clinical swallowing examination. Therefore the management of dysphagia on the ICU cannot be guided solely by clinical tools.
Fiberoptic Endoscopic Evaluation of Swallowing (FEES)
During FEES a flexible naso-pharyngo-laryngoscope is introduced transnasally into the pharynx for direct visualisation of the swallowing act.
FEES aims to:
(i) identify pathological movement patterns;
(ii) evaluate the effectiveness and safety of the swallow process, and
(iii) recommend appropriate food consistencies as well as special diets or swallowing techniques on an individual basis.
Available data indicate that FEES is a welltolerated and safe examination. In 6,000 investigations only 222 (3.7%) had to be stopped at the patient's request (Langmore 2001). The most commonly reported side effect was selflimited nosebleed being present in approximately 1% of cases. More serious events like vasovagal syncope and laryngospamus occurred in 0.03% (Aviv et al. 2000; Aviv et al. 2001; Cohen et al. 2003). These results were replicated in a group of acute stroke patients. Although the rate of selflimited nosebleed was with 6% higher than in the other studies, no serious side effects were reported, and vegetative symptoms like heart rate and blood pressure fluctuations were mild (Warnecke et al. 2009a). Meanwhile numerous studies have shown that FEES is equivalent to the historic gold standard, the videofluoroscopy (VFSS=Videofluoroscopic Swallow Study) in detecting the most important critical findings like aspiration and residues (Wu et al. 1997; Kelly et al. 2006; Kelly et al. 2007). FEES is also an extremely reliable method, which is underlined by an interrater consensus of over 90% in various studies (Leder et al. 1998; Dziewas et al. 2008).
On the ICU the essential practical advantages of FEES over VFSS are:
• the examination can be done at the bedside, and patients with highly restricted motor functions as well as bedridden or uncooperative patients can be examined.
• repeated follow-up examinations are safely possible without the issue of radiation exposure.
• saliva management can be assessed directly (Langmore 2003).
As has been shown in a large observational study, FEES in daily practice on the ICU is indeed helpful to assess airway protection and to steer dysphagia management (Hafner et al. 2008). Altogether 913 endoscopic swallowing evaluations were performed in 553 patients over a period of 45 months at several intensive care units. Based on the result of FEES, 6.3% of the patients were tracheotomised to protect the airway, 49.7% received a feeding tube and 13.2% a PEG to ensure enteral feeding. In 30.7% of patients oral diet was judged to be feasible. Two other studies showed that in acute stroke the endoscopic evidence of saliva aspiration is a strong predictor for the need for intubation later on (Dziewas et al. 2008; Warnecke et al. 2009b). These results underline the need for early instrumental dysphagia assessment in the critically ill.
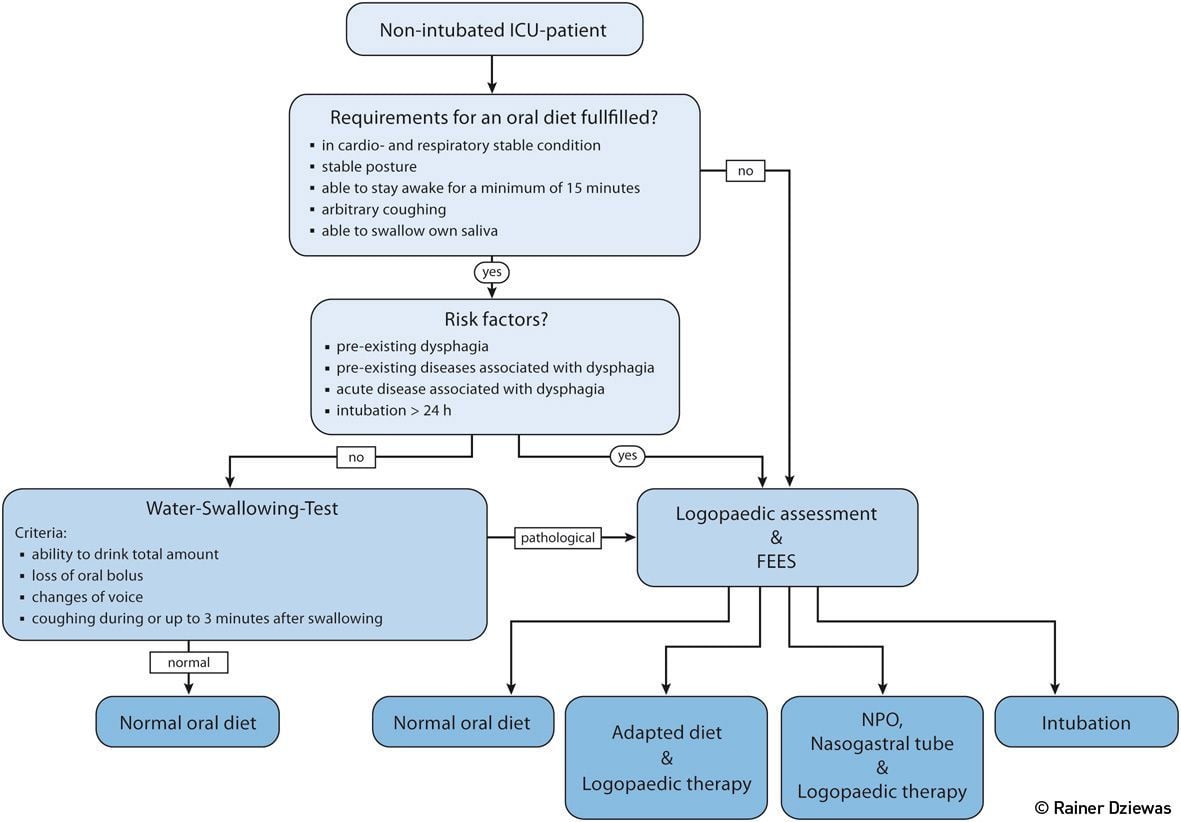
Figure 3. Diagnostic Algorithm for the Assessment of Dysphagia in Non-Intubated ICU-Patients
Diagnostic Algorithms for the Management of Dysphagia in Non-Intubated and Trachotomised ICU Patients
The Non-Intubated Patient
In non-intubated ICU patients dysphagia assessment provides important information for the selection of the appropriate diet and also guides the initiation of further protective and rehabilitative measures. Although there is currently no standardised algorithm that has been evaluated in prospective studies, the one proposed in Figure 3 considers the advantages and disadvantages of the various diagnostic modalities and implements existing knowledge in order to give pragmatic recommendations. First, minimum basic requirements for an oral diet such as a sufficient state of vigilance and trunk stability are evaluated. Next, the risk factors for dysphagia are assessed. Apart from the patient’s main diagnosis specific co-morbidities need to be considered. Since dysphagia is at least in part frequently a side effect of the ICU treatment itself, the duration of intubation and artificial ventilation with a cut-off value of 24 hours is introduced as an additional criterion. In case there are none of these risk factors present, for example in a patient with an uncomplicated surgery followed by quick extubation, it is sufficient to carry out a simple aspiration screening. If this test is normal the patient may directly get an oral diet. If at any of the three steps the just described indicators of dysphagia are present, a clinical swallowing examination by a speech therapist and, ideally, a FEES should be performed. With the help of these diagnostic procedures a decision whether the patient can receive a normal oral diet, requires a special consistency-adapted diet, is in need of tube feeding or should be considered as a candidate for intubation to secure the airway can be made.
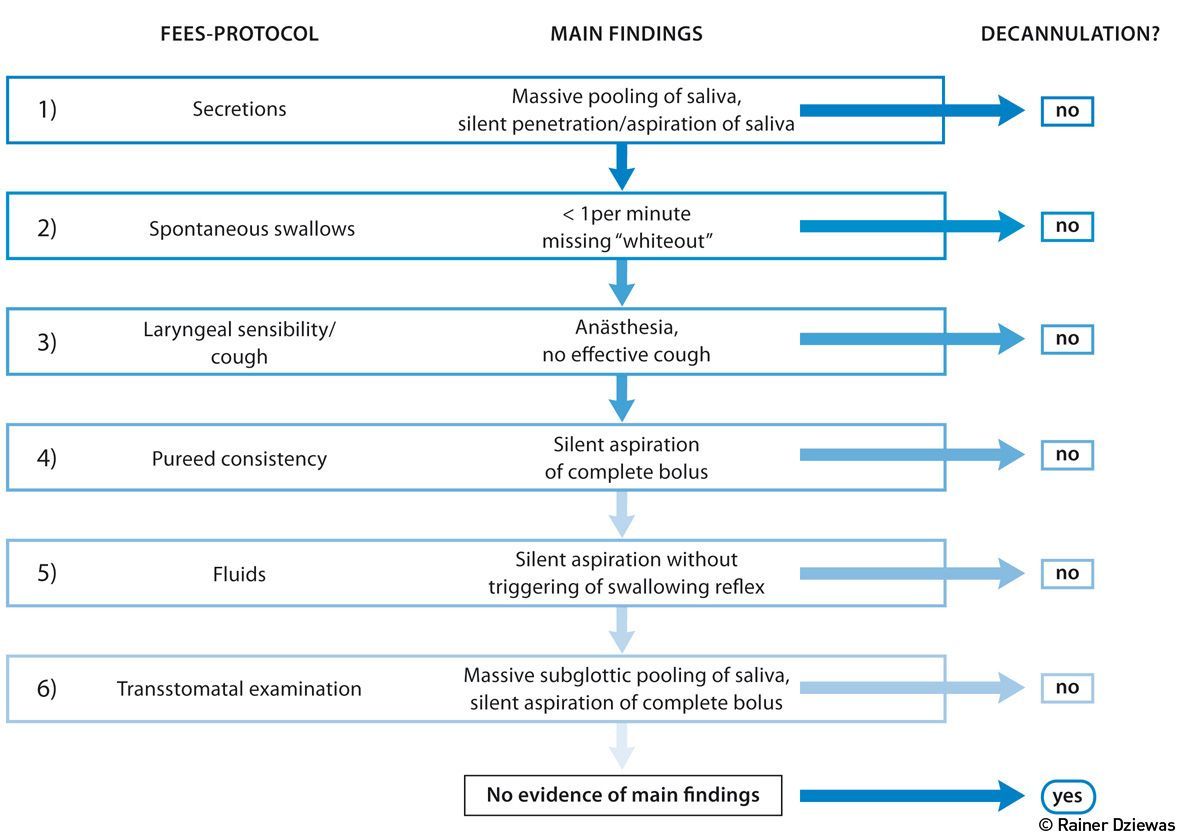
The Tracheotomised Patient
The tracheostomy, in particular the minimally invasive dilatational approach, is now a standard procedure on most ICUs, and the majority of long-term ventilated patients are ventilated through this airway access. After successful weaning from the respirator the question arises whether the removal of the tracheal cannula can be achieved. Due to the limitations of the clinical swallowing examination assessment of the swallowing function in this context should include FEES (Warnecke and Dziewas 2013). To increase the reliability and reproducibility of the endoscopic examination, a standardised, step-by-step approach might be implemented (Warnecke et.al. 2013) (see Figure 4). After suctioning pharyngeal secretions and deflating the tracheal cuff the extent and localisation of salivary retentions are assessed and the spontaneous swallowing frequency is observed. If massive pooling or silent aspiration of saliva is visible (step 1), the investigation is stopped at this point. If not, the number and efficiency of spontaneously occurring swallows is rated for at least two minutes (step 2). If more than one efficient swallow per minute occurs, the investigation proceeds and laryngeal sensibility and cough reflex are tested by gently touching the aryepiglottic region with the tip of the endoscope (step 3). Patients demonstrating an efficient cough are given a teaspoon of purée consistency (step 4). If no aspiration occurs, the patient is given a teaspoon of coloured water (step 5). Silent aspiration of the water, without triggering the swallowing reflex, also indicates lack of readiness for decannulation; otherwise, having swallowed successfully, the patient is regarded as being able to sufficiently protect his/her airway and the tracheostomy tube may be removed immediately. After that, the endoscope is briefly inserted through the stoma, flexed upward to visualise the subglottic structures and downward to inspect the lower trachea, in order to ensure that there are no structural abnormalities comprising the airway (Donzelli et al. 2001). The application of this algorithm in 100 tracheotomised patients who were weaned from the ventilator on a neurological ICU allowed safe decannulation in more than half of the patients (Warnecke et al. 2013). In the further course of treatment only one patient had to be recannulated. Noteworthy also was that the clinical swallowing examination, which took into account the parameters state of vigilance, cooperation skills, saliva swallowing, coughing and amount of collected saliva from the tracheal cannula, would have allowed decannulation in only 27 patients.
Acknowledgements
This article has been modified and shortened compared to Dziewas R, Glahn J (2015) Schluckstörungen auf der Intensivstation. In: NeuroIntensiv. Heidelberg: Springer, pp: 108-14. All Figures were designed by Heike Blum, Department of Neurology, University Hospital Münster, Germany.
References:
Aviv JE, Kaplan ST, Langmore SE (2001a). The safety of endoscopic swallowing evaluations. In: Langmore SE. Endoscopic evaluation and treatment of swallowing disorders. New York: Thieme, pp. 235-42.
Aviv JE, Kaplan ST, Thomson JE et al. (2000) The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST ): an analysis of 500 consecutive evaluations. Dysphagia, 15(1): 39-44.
Barquist E, Brown M , Cohn S et al. (2001) Postextubation fiberoptic endoscopic evaluation of swallowing after prolonged endotracheal intubation: a randomized prospective trail. Crit Care Med, 29(9): 1710-3.
Bours GJ, Speyer R, Lemmens J et al. (2009) Bedside screening tests vs. videofluoroscopy or fiberoptic endoscopic evaluation of swallowing to detect dysphagia in patients with neurological disorders: a systematic review. J Adv Nurs 65(3): 477-93.
Cassier-Woidasky AK, Nahrwold J, Glahn J (2012) Pflege von Patienten mit Schlaganfall; von der Stroke Unit bis zur Rehabilitation, Stuttgart: W. Kohlhammer.
Cohen MA, Setzen M, Perlman PW (2003) The safety of flexible endoscopic evaluation of swallowing with sensory testing in an outpatient otolaryngology setting. Laryngoscope, 113(1): 21-4.
Donzelli J, Brady S, Wesling M et al. (2001) Simultaneous modified Evans blue dye procedure and video nasal endoscopic evaluation of the swallow. Laryngoscope, 111(10): 1746-50.
Dziewas R, Warnecke T, Oelenberg S et al. (2008) Towards a basic endoscopic assessment of swallowing in acute stroke - development and evaluation of a simple dysphagia score. Cerebrovasc Dis, 26(1): 41-7.
Dziewas R, Glahn J (2015) Schluckstörungen auf der Intensivstation. Neurointensiv, Stuttgart: Thieme, pp. 108-14.
El Solh A, Okada M, Bhat A et al. (2003) Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med, 29(9): 1451-5.
Gross RD, Atwood CW, Ross SB et al. (2009) The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 179(7): 559-65.
Hafner G, Neuhuber A, Hirtenfelder S et al. (2008) Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. Eur Arch Otorhinolaryngol, 265(4): 441-6.
Hales PA, Drinnan MJ, Wilson JA (2008) The added value of fibreoptic endoscopic evaluation of swallowing in tracheostomy weaning. Clin Otolaryngol, 33(4): 319-24.
Kelly AM, Drinnan MJ, Leslie P (2007) Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope, 117(10): 1723-7.
Kelly AM, Leslie P, Beale T et al. (2006) Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol, 31: 425-32.
Langmore SE (2001) Endoscopic evaluation and treatment of swallowing disorders. New York: Thieme.
Langmore SE (2003) Evaluation of oropharyngeal dysphagia: which diagnostic tool is superior? Curr Opin Otolaryngol Head Neck Surg, 11(6): 485-9
Leder SB, Sasaki CT, Burrell MI (1998) Fiberoptic endoscopic evaluation of dysphagia to identify silent aspiration. Dysphagia, 13(1): 19-21.
Macht M, Wimbish T, Bodine C et al. (2013) ICU-acquired swallowing disorders. Crit Care Med, 41(10): 2396-405.
Macht M, Wimbish T, Clark BJ et al. (2011) Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care, 15(5): R231.
McCullough GH, Wertz RT, Rosenbeck JC et al. (2000) Interand intrajudge reliability of a clinical swallowing examination of swallowing in adults. Dysphagia, 15(2): 58-67.
McCullough GH, Wertz RT, Rosenbek JC (2001) Sensitivity and specificity of clinical /bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord, 34(1-2): 55-72.
Noordally SO, Sohawon S, De Gieter M et al. (2011) A study to determine the correlation between clinical, fiber-optic endoscopic evaluation of swallowing and videofluoroscopic evaluations of swallowing after prolonged intubation. Nutr Clin Pract, 26(4): 457-62.
Shaker R, Li Q, Ren J et al. (1992) Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol, 263(5 Pt 1): G750-5.
Skoretz SA, Flowers JL, Martino R (2010) The incidence of dysphagia following endotracheal intubation: a systematic review. Chest, 137(3): 665-73.
Warnecke T, Dziewas R (2013) Neurogene Dysphagien. Diagnostik und Therapie. Stuttgart: Kohlhammer.
Warnecke T, Ritter M, Kroger B et al. (2009b) Fiberoptic endoscopic dysphagia severity scale predicts outcome after acute stroke. Cerebrovasc Dis, 28(3): 283-9.
Warnecke T, Suntrup S, Teismann IK et al. (2013) Standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients. Crit Care Med, 41(7): 1728-32.
Warnecke T, Teismann I, Oelenberg S et al. (2009a) The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke, 40(2): 482-6.
Wu CH, Hsiao TY, Chen JC et al.(1997) Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope, 107(3): 396-401