ICU Management & Practice, ICU Volume 15 - Issue 3 - 2015
The Case for Liberal Glycaemic Management
Background
Several large randomised
controlled trials (RCTs) have helped provide evidence to guide clinicians’ decisions
about blood glucose management in critically ill patients. In two landmark single
centre studies, investigators from Leuven reported a reduction in mortality or
morbidity with tight glycaemic control (80-110 mg/dl or 4.4-6.1 mmol/L) in
critically ill patients (Van den Berghe et al. 2001; 2006). However, the findings
of five more recent intensive care-based RCTs, including the very large
(>6,000 patients) NICE-SUGAR trial, were unable to replicate these findings
(Finfer et al. 2009; Brunkhorst et al 2008; De La Rosa et al 2008; Arabi et al
2008), and in the case of NICE-SUGAR they actually found harm with tight
glycaemic control. The aggregate findings of such trials have therefore led to
recommendations to target blood glucose levels below 180 mg/dl (10 mmol/L) in
order to avoid excessive hyperglycaemia and minimise the risk of hypoglycaemia.
Despite the above consensus,
it remains unclear why these trials delivered such disparate and contradictory
results. A possible explanation for the discordant findings of glycaemic
control trials might relate to the high rate of use of total parenteral
nutrition in the Leuven studies (Marik and Preiser 2010; Egi et al. 2011).
Given that parenteral nutrition is uncommon in contemporary intensive care
units, however, this possibility makes the Leuven studies of unclear modern significance.
More importantly, and of relevance to this article, all these randomised
studies considered diabetic and non-diabetic patients in the same cohort, and
treated such patients in the same way, creating the potential for differing outcomes,
because of the variable proportion of such patients and their pre-admission
treatment. This uniform approach to glycaemic control in patients with or
without diabetes mellitus (DM) may have stemmed from the notion that, even though
pre-existing DM has been identified as a risk factor for the development of
critical illness, patients with DM have comparable mortality rates to those
without diabetes (Stegenga et al. 2010; Vincent et al. 2010), and no specific
data existed at that time to suggest that they should be considered unique from
the point of view of glycaemic management. Thus general ICU glycaemic control
studies have until now influenced the management of glycaemia equally in DM and
non-DM patients admitted to ICU. However, this may be unwise.
Current Practice in Diabetic Patients
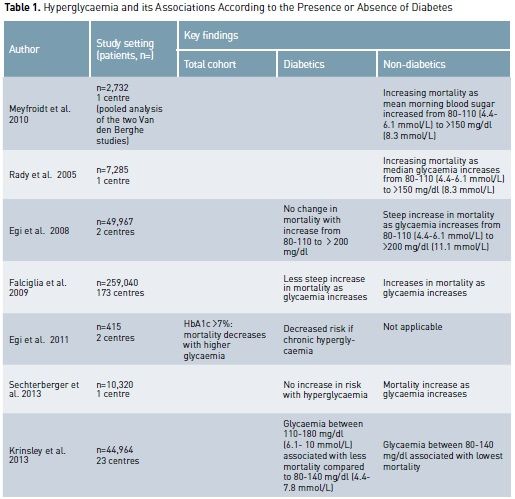
Because of the above trials, current guidelines recommend targeting blood glucose levels of 140 to 180 mg/dl (7.8 to 10 mmol/L) in all critically ill patients (American Diabetes Association 2012; Ichai et al 2010). These recommendations appear justified not only by the findings of RCTs, but also by multiple observational studies that have demonstrated a higher mortality risk with both hyper- and hypo-glycaemia (Falciglia et al. 2009; NICE-SUGAR investigators 2012; Joeren et al. 2010). We speculate, however, that the ideal glycaemic range might differ in patients with DM, and moreover, even within such a cohort, it may depend on pre-existing glycaemic control. As DM patients comprise approximately 20% of all ICU admissions (Soulimane et al. 2012, Cely et al 2004), the issue of optimal glycaemic control in such patients appears clinically important.
It is important to reflect
that in addition to patients with known diabetes, there are other patients
admitted in ICU, who have chronic impairment in glycaemia (as shown by HbA1c levels),
but in whom a diagnosis of diabetes had not been made prior to hospital
presentation. Such patients with “unrecognised diabetes” at ICU admission may
also require a specific level of glycaemic control similar to that of patients with
known DM. To illustrate this point, in 2014 Plummer et al. described the
prevalence of “unrecognised diabetes” in an Australian tertiary hospital as
5.5% of all ICU admissions (Plummer et al. 2014). However, an even higher value
of 15% of ICU admissions was reported by a study conducted in Croatia (Gornik
et al. 2010), and two American studies (Cely et al. 2004, Hoang et al. 2014)
recorded “unrecognised diabetes” in 12% and 13.7 % of ICU admissions
respectively. Thus patients with chronic hyperglycaemia (known diabetes and unrecognised
diabetes) prior to ICU admission may represent up to 30% of all ICU admissions.
Why Consider a Different Approach to Glycaemic Management Strategy in Diabetic Patients?
The
Meaning of Hyperglycaemia
Critically ill patients are
at high risk of developing stress hyperglycaemia (Dungan et al. 2009), which is
characterised by a high state of hepatic gluconeogenesis, excessive insulin resistance
and increase of circulating cytokines, cortisol, epinephrine and glucagone. In
patients with diabetes, chronic hyperglycaemia may already exist, and
critically ill patients with DM may simply display slightly or moderately worse
glucose derangements. Among non-diabetic patients, stress hyperglycaemia is
associated with an increased risk of mortality after adjustment for illness
severity. In particular the risk of death increases in proportion to blood
glucose levels (Falciglia et al. 2009; Krinsley et al. 2003; Umpierrez et al.
2002; Finney et al. 2003). In contrast, data from several studies suggest that
the adverse outcomes associated with hyperglycaemia are negligible or absent in
patients with pre-existing diabetes (Sechterberger et al. 2013; Krinsley et al.
2013; Egi et al. 2008; Egi et al. 2011, Rady et al. 2005).
In all the studies cited
above, a strong association between hyperglycaemia (episodes of hyperglycaemia and
time-weighted hyperglycaemia) and ICU mortality was consistently found in non-diabetic
patients but not in diabetic patients. This lack of association between
hyperglycaemia and mortality may relate to the fact that diabetic patients have
developed tolerance to hyperglycaemia. Thus diabetic patients may behave in
relation to glucose the way patients with chronic obstructive pulmonary disease
behave in relation to the correction of chronic hypoxaemia (Joosten et al.
2007), or patients with chronic hyponatraemia behave in relation to the
correction of chronic hyponatraemia (Widdess-Walsh et al. 2007), and
hypertensive patients relate to the rapid correction of high blood pressure
levels (Shuaib et al.1992).
To further understand the
importance of chronic hyperglycaemia in diabetic patients and its impact on
acute glycaemic management, it is important to appreciate the value of
measuring HbA1c. There is a direct relationship between HbA1c and mean
glycaemia, because haemoglobin remains glycated during the approximate 120-day
lifespan of the erythrocytes. As such a HbA1c of 6% corresponds to a mean
plasma glucose level of 7.5 mmol/L (126 mg/dl) in the previous six to nine
weeks, and each 1% increase in HbA1c corresponds to an increase of about
2mmol/L in mean plasma glucose levels (Peterson et al. 1998; Rohlfing et al.
2002). By measuring HbA1 in diabetic patients on admission, it is possible to
estimate typical glucose levels, and theoretically it should then be possible
to aim for type-individualised care (a kind of precision medicine) that seeks
to maintain individual homeostasis by aiming for an acute glucose target, which
approximates the normal glycaemic control for that diabetic patient prior to
ICU admission.
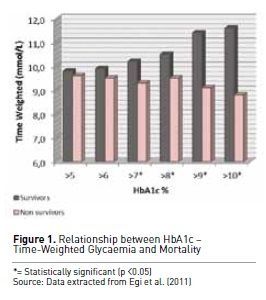
To further understand this
concept, Egi et al. (2011) evaluated in detail the interaction between
pre-morbid hyperglycaemia, measured by means of HbA1c levels at ICU admission,
acute glycaemia as delivered in ICU and hospital mortality. Their findings
showed that patients with higher pre-admission HbA1c levels (>7%) were much
less likely to die in hospital when their mean glucose concentrations in ICU
were >180 mg/dl (10 mmol/L) (see Figure 1). These data support the concept that
in the presence of chronic hyperglycaemia (Egi et al. 2011) tolerating higher
than normal blood glucose levels can be considered safe and may even be
potentially desirable in diabetic patients experiencing hyperglycaemia in the ICU.
In critically ill diabetic
patients any consistent difference between premorbid glycaemia and mean
glycaemia during ICU admission in the direction of decreased glucose levels
could be considered a form of “relative hypoglycaemia”. Thus if a patient is used
to having, for example, a 180mg/dl (10 mmol/L) blood glucose level, then such a
patient may experience the physiological stress of hypoglycaemia at 100 mg/dl (5.5
mmol/L) of blood glucose concentration. Such stress may then be the same as
that of a normal person who develops a glucose level of <40 mg/dl (2.2
mmol/L). Such “relative hypoglycaemia” may be a silent contributor to morbidity
and mortality in diabetic patients, especially in a context (ICU) where
symptoms of “relative hypoglycaemia” are often masked by sedative or analgesic
medication.
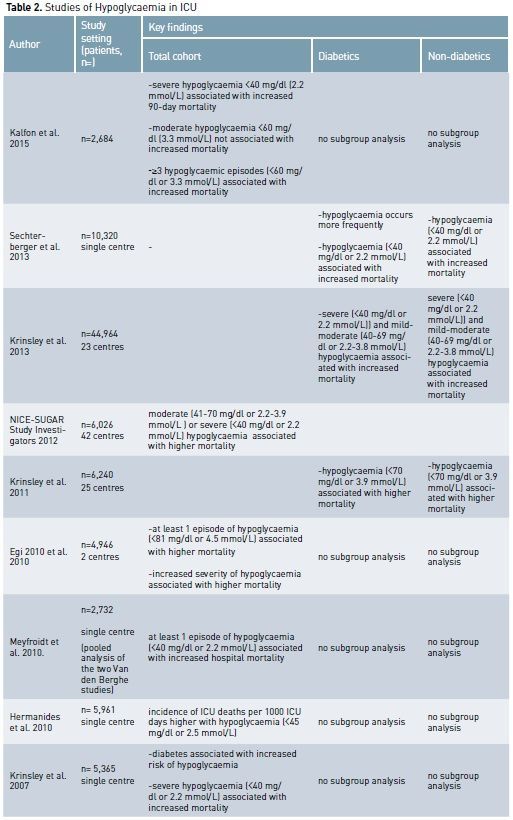
The Importance of Hypoglycaemia
Hypoglycaemia has deleterious effects in critically ill patients by increasing the systemic inflammatory response (Dotson et al 2008), inducing neuroglycopaenia (Schlenk et al. 2008), inhibiting the corticosteroid response to stress (Keller-Wood et al. 1983), impairing sympathetic system responsiveness (Herlein et al. 2006) and causing cerebral vasodilatation (Dieguez et al. 1997). Hypoglycaemia, both severe (<40 mg/dl or 2.2 mmol/L) and mild/ moderate (less than 70 mg/dl or 3.9 mmol/L), likely contributes to increased mortality in critically ill diabetics and non-diabetic patients. (Kalfon et al. 2015; NICE-SUGAR investigators 2012; Hermanides et al. 2010).
It seems biologically reasonable to think that hypoglycaemia during critical illness in diabetic patients should be considered in the context of chronic overall glycaemic control. The main aim in DM patients may then be not only to avoid absolute hypoglycaemia, but also to avoid “relative hypoglycaemia”, which can be expressed by the “distance” or decrease between chronic glycaemia levels and the blood glucose levels experienced during acute illness. As described above, relative hypoglycaemic episodes for diabetic patients might have the same biological toxicity that absolute hypoglycaemic conditions produce in non-diabetic patients.
Moreover the ‘protective’
modifications associated with chronic hyperglycaemia leave cells vulnerable to
relative glycopaenia, particularly in situations where hypotension and hypoxia co-exist.
Some evidence suggests that chronic hyperglycaemia sets up a pattern of
cellular conditions that might actually protect against acute hyperglycaemia-mediated
damage but exacerbate hypoglycaemia-induced injury. One mechanism for this
effect might be the preferential ‘down regulation’ of insulin independent
glucose transporters under chronic hyperglycaemic conditions (Deane and
Horowitz 2013).
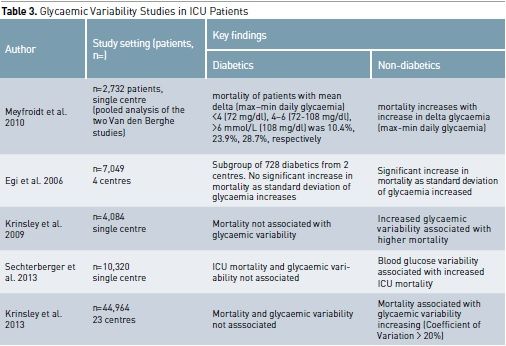
The Importance of Glycaemic Variability
To define the best glycaemic
management in diabetic patients, it is therefore logically necessary to take
into account that it is relative dysglycaemia that may require treatment
instead of just absolute hypoglycaemia or hyperglycaemia. Dysglycaemia can be seen to encompass three domains
of glycaemic control: hypoglycaemia, hyperglycaemia and glycaemic variability.
(Egi et al. 2007). Therefore a trend measure like glycaemic variability has
been defined as the standard deviation of mean glucose level during ICU stay or
as the difference between highest and lowest glycaemia in an established time
period. Diabetic patients have greater absolute glycaemic variability than
non-diabetic patients, and this might also affect the different association
between glycaemia and outcome (Krinsley et al. 2009).
There is a growing body of
evidence to suggest that for non-diabetic patients marked fluctuations in blood
glucose are also related to an increased risk of mortality (Ali et al 2008;
Meyfroidt et al. 2010; Krinsley et al. 2008; Dossett et al. 2008; Krinsley et
al. 2009; Egi et al. 2006). A 2003 study found that in umbilical vein cells
glycaemic fluctuations caused a higher level of oxidative stress compared to
sustained hyperglycaemia (Quagliaro et al. 2003). This correlation has been demonstrated
also in patients with type 2 diabetes. Such increased oxidative stress can
result in endothelial dysfunction and can contribute to vascular damage
(Monnier et al. 2006).
Despite these concerns, the
impact of glycaemic variability in critically ill diabetic patients has not been
extensively investigated. In fact, a large study demonstrated that glycaemic
variability had an independent association with mortality among non-diabetic
individuals but not among diabetics (Krinsley et al. 2009). Such a finding
supports the concept that a degree of spontaneous tolerance to glycaemic
variability may occur in diabetic patients due to their glycaemic history prior
to ICU admission, which may have included chronic exposure to much greater
levels of glycaemic variability than non-diabetic patients.
Thus efforts to maintain
glycaemia in the normal range and decrease variability may be potentially injurious
for patients with altered premorbid glycaemia. In this regard, a recent study
demonstrated that a higher level of Time In Range glycaemia (TIR) (70mg/dl-140
mg/dl or 3.9-7.8 mmol/L) had a positive correlation with decreased mortality in
a population of non-diabetic critically ill patients, while, among patients
with diabetes, there was no consistent relationship between TIR and mortality
(Krinsley et al. 2015).
Chronic Glucose Management in Diabetes and its Relevance to Critical Illness
Diabetes is mainly a chronic
disease, and so lessons may also be derived from its “chronic” treatment, which
have potential relevance to its acute management. For example, the Action to
Control Cardiovascular Risk in Diabetes (ACCORD) study reported that in type 2
diabetic individuals in the ambulatory setting, aggressive glucose control to
reduce glycosylated haemoglobin level from 8.3 to 6.4% over just a few months
significantly increased mortality compared with control therapy. This means
that a reduction of mean glycaemia of about 0.7 mmol/L per month appeared to be
injurious. In contrast, the Action in Diabetes and Vascular Disease (ADVANCE
study), published at the same time, showed that attempting to reduce
glycosylated haemoglobin levels over a much longer period of time (slower
decrease in glycaemia which allowed adaptation) was associated with a
non-significant downward trend in mortality (Advance Collaborative Group et al.
2008; Gerstein et al. 2008; Dluhy et al. 2008 ).
There were important differences
between ACCORD and ADVANCE in terms of blood glucose, which may be relevant to
acute glycaemic management. Firstly, the baseline level of HbA1 was different:
ACCORD included patients with a mean HbA1c of 8.1% compared with 7.5% in the
ADVANCE study. Secondly, the speed of lowering HbA1c was very different: in the
ACCORD study, the HbA1c levels fell to 6.7% within the first 4 months while in
ADVANCE the HbA1c decreased to 7% within the first 6 months (a big difference in
speed of reduction - such that the rate of decrease in glycaemia was almost 10 times
faster in ACCORD). These findings in the chronic setting, showing an
association between increased mortality and fast reduction in HbA1c levels,
raise concerns that any therapy that leads to a rapid decrease in glycaemia in diabetic
patients with high premorbid HbA1c values is dangerous and should be avoided. As
such, normalisation of glycaemia in ICU patients with DM and chronic
hyperglycaemia may indeed be dangerous.
Conclusions
On the basis of the modern
“three domains” paradigm of glycaemic control in the acute setting, diabetic
patients are different. In such patients, hyperglycaemia and greater glycaemic variability
are not independently associated with increased mortality. Moreover the third
domain of hypoglycaemia also appears different. In particular, in diabetic
patients with poor pre-admission glycaemic control (chronic hyperglycaemia), even
acute normoglycaemia may actually be a form of relative hypoglycaemia and may
be associated with increased mortality. The evidence suggests that glycaemic
management algorithms should be tailored differently for diabetic and non-diabetic
patients and that a more liberal set of targets (”permissive moderate
hyperglycaemia”) may be justified in diabetic patients. Finally it appears
desirable that further studies should investigate optimal blood glucose targets
for critically ill diabetic patients in relation to premorbid glycaemia to test
the feasibility and safety as well as the possible efficacy of a degree of
“permissive moderate hyperglycaemia”.
References:
ADVANCE Collaborative Group, Patel A, MacMahon S et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med, 358(24): 2560-72.
Ali NA, O’Brien JM Jr, Dungan K et al. (2008) Glucose variability and mortality in patients with sepsis. Crit Care Med, 36(8): 2316-21.
American Diabetes Association (2012) Standards of medical care in diabetes, Diabetes Care, 35(Suppl 1): S11–63.
Arabi YM, Dabbagh OC, Tamim HM et al. (2008) Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med, 36(12): 3190-7.
Brunkhorst FM, Engel C, Bloos F et al. (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med, 358(2): 125–39.
Cely CM, Arora P, Quartin AA et al. (2004) Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness, Chest, 126(3): 879–87.
Deane AM, Harowitz M (2013) Disglycaemia in critically ill- significance and management. Diabetes Obes Metab, 15(9): 792–801.
De La Rosa GDC, Donado JH, Restrepo AH et al. (2008) Grupo de Investigacion en Cuidado intensivo: GICI-HPTU. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care, 12(5): R120.
Diéguez G, Fernández N, García JL et al. (1997) Role of nitric oxide in the effects of hypoglycemia on the cerebral circulation in awake goats. Eur J Pharmacol, 330(2-3): 185–93.
Dluhy RG, McMahon GT (2008) Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med, 358(24): 2630–3.
Dossett LA, Cao H, Mowery NT et al. (2008) Blood glucose variability is associated with mortality in the surgical intensive care unit. J Am Surg, 74(8): 679-85.
Dotson S, Freeman R, Failing HJ et al. (2008) Hypoglycemia increases serum inter- leukin-6 levels in healthy men and women. Diab Care, 31(6): 1222–3.
Egi M, Finfer S, Bellomo R (2011) Glycemic Control in the ICU. Chest, 140(1): 212–20.
Egi M, Bellomo R, Stachowski E et al. (2010) Hypoglycaemia and outcome in critically ill patient. Mayo Clin. Proc. 85(3): 217-24.
Egi M, Bellomo R, Stachowski E et al. (2008) Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med, 36(8): 2249-55.
Egi M, Bellomo R, Stachowski E et al. (2011) The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med, 39(1): 105–111.
Egi M, Bellomo R, Stachowski E et al. (2007) Circadian rhythms of blood glucose values in critically ill patients. Crit Care Med, 35(2): 416-21.
Egi M, Bellomo R, Stachowski E et al. (2006) Variability of blood glucose concentration and short-term mortality of critically ill patients. Anesthesiology, 105(2): 244–52.
Falciglia M, Freyberg RW, Almenoff PL et al. (2009) Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med, 37(12): 3001-9.
Finfer S, Chittbock DR, Su SY et al. (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med, 360(13): 1283–97.
Finney SJ, Zekveld C, Elia A et al. (2003) Glucose control and mortality in critically ill patients. JAMA, 290(15): 2041–7.
Gerstein HC, Miller ME, Byington RP et al. (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med, 358(24): 2545–59.
Gornik I, Vujaklija-Brajkovic A, Renar IP et al. (2010) A prospective observational study of the relationship of critical illness associated hyperglycaemia in medical ICU patients and subsequent development of type 2 diabetes. Crit Care, 14(4): R130.
Herlein JA, Morgan DA, Phillips BG et al. (2006) Antecedent hypoglycemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology, 147(6): 2781–8.
Hermanides J, Bosman RJ, Vriesendorp TM et al. (2010) Hypoglycemia is associated with intensive care unit mortality. Crit Care Med, 38(6): 1430-4.
Hoang QN, Pisani MA, Inzucchi S et al. (2014) The prevalence of undiagnosed diabetes mellitus and the association of baseline glycemic control on mortality in the intensive care unit: a prospective observational study. J Crit Care, 29(6): 1052–6.
Ichai C, Preiser JC, Société Française d'Anesthésie-Réanimation et al. (2010) International recommendations for glucose control in adult non diabetic critically ill patients. Crit Care, 14(5): R166.
Joosten SA, Koh MS, Bu X et al. (2007) The effects of oxygen therapy in patients presenting to an emergency department with exacerbation of chronic obstructive pulmonary disease. Med J Aust, 186(5): 235–8.
Kalfon P, Le Menach Y, Ichai C et al. (2015) Severe and multiple hypoglycemic episodes are associated with increased risk of death in ICU patients. Crit Care, 19:153.
Keller-Wood ME, Shinsako J, Dallman MF (1983) Inhibition of the adrenocorticotropin and corticosteroid responses to hypoglycemia after prior stress. Endocrinology, 113(2): 491–6.
Krinsley JS (2003) Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc, 78(12): 1471–18.
Krinsley JS, Grover A (2007) Severe hypoglycemia in critically ill patients : risk factors and outcomes. Crit Care Med 35(10): 2262 – 7.
Krinsley JS, Schultz MJ, Sprong PE et al. (2011) Mild hypoglycemia is independently associated with increased mortality in critically ill patients. Crit Care, 15(4): R173.
Krinsley JS, Egi M, Kiss A et al. (2013) Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care, 17(2): R37.
Krinsley JS (2009) Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diab Sci Tech, 3(6): 1292–1301.
Krinsley JS (2008) Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med, 36(11): 3008-13.
Krinsley JS, Preiser JC (2015) Time in blood glucose range 70 to 140 mg/dl > 80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care, 19: 179.
Marik PE, Preiser JC (2010) Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest, 137(3): 544-51.
Meyfroidt G, Keenan DM, Wang X et al. (2010) Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med, 38(4): 1021-9.
Monnier L, Mas E, Ginet C et al. (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA, 295(14): 1681-7.
NICE-SUGAR Study Investigators, Finfer S, Liu B et al. (2012) Hypoglycemia and risk of death in critically ill patient. N Engl J Med, 367(12): 1108-18.
Peterson KP, Pavlovich JG, Goldstein D et al. (1998) What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem, 44(9): 1951–8.
Plummer MP, Bellomo R, Cousins CE et al. (2014) Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med, 40(7): 973–80.
Preiser JC, Devos P, Ruiz-Santana S et al. (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the glucontrol study. Intensive Care Med, 35(10): 1738–48.
Quagliaro L, Piconi L, Assaloni R et al. (2003) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes, 52(11): 2795-804.
Rady MY, Johnson DJ, Patel BM et al. (2005) Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc, 80(12): 1558-67.
Rohlfing CL, Wiedmeyer HM, Little RR et al. (2002) Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care, 25(2): 275–8.
Schlenk F, Graetz D, Nagel A et al. (2008) Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care, 12(1): R9.
Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM et al. (2013) The effect of diabetes mellitus on the association between measures of glycemic control and intensive care mortality: a retrospective cohort study. Crit Care, 17(2): R52.
Shuaib A (1992) Alteration of blood pressure regulation and cerebrovascular disorders in the elderly. Cerebrovasc Brain Metab Rev, 4(4): 329–45.
Soulimane S, Simon D, Shaw J et al. (2012) HbA1c, fasting plasma glucose and the prediction of diabetes: Inter99, Aus Diab and D.E.S.I.R. Diabetes Res Clin Pract, 96(3): 392–9.
Stegenga ME, Vincent JL, Vail GM et al. (2010) Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med, 38(2): 539–45.
Umpierrez GE, Isaacs SD, Bazargan N et al.(2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab, 87(3): 978–82.
van den Berghe G, Wouters P, Weekers F et al. (2001) Intensive insulin therapy in the critically ill patients. N Engl J Med, 345(19): 1359-67.
van den Berghe G, Wilmer A, Hermans G et al. (2006) Intensive insulin therapy in the medical ICU. N Engl J Med. 2006, 354(5): 449-61.
Vincent JL, Preiser JC, Sprung CL et al. (2010) Insulin-treated diabetes is not associated with increased mortality in critically ill patients. Crit Care, 14(1): R12.
Widdess-Walsh P, Sabharwal V, Demirjian S et al. (2007) Neurologic effects of hyponatremia and its treatment. Cleve Clin J Med, 74(50: 377–83.









