ICU Management & Practice, ICU Volume 14 - Issue 2 - Summer 2014
Authors

Alain Broccard, MD, FCCP
Professor of Medicine
Division of Pulmonary, Allergy,
Critical Care and Sleep Medicine, University of Minnesota
Medical Director of Critical Care and Respiratory Services
Fairview Southdale Hospital Edina, MN, USA
[email protected]

Damien Tagan, MD, MHA
Lecturer, Lausanne University
Head of Medical Intensive Care Unit, Department of Internal Medicine
Riviera- Chablais Hospital Vevey, Switzerland
[email protected]
Point-of-care ultrasonography allows intensivists to non-invasively assess diaphragmatic function. We review here the key concepts and technical aspects needed to assess diaphragmatic dysfunction in the ICU and allow intensivists with prior ultrasound training to answer clinical questions related to the diaphragm. We anticipate that this and other novel applications of point-of-care ultrasound will continue to grow in the years to come.
Introduction
Point-of-care ultrasonography (POCU) has changed the practice of emergency and critical care medicine. Until recently the emphasis has mainly been on using POCU to assist with percutaneous procedures and/or with the diagnosis and management of life-threatening conditions such as trauma, shock, pulmonary oedema or pneumothorax. More recently, new but not widely embraced ICU applications for POCU have emerged, which can better address important ICU clinical questions such as lung recruitability or diaphragmatic function.
Diaphragmatic dysfunction may lead to the need for mechanical ventilation, contribute to failure to wean from it or, as recently demonstrated, be the consequence of the ventilator strategy/mode applied (Powers et al. 2013). Bedside assessment of diaphragmatic function by physical exam is insensitive, while measurement of negative inspiratory pressure is effort-dependent. Alternative methods based on phrenic nerve stimulation, diaphragmatic electromyography or transdiaphragmatic pressure measurements are not widely available outside the research arena. The limited ability to identify diaphragmatic dysfunction in the ICU has likely caused underestimation of its contribution to the morbidity of ventilated patients. POCU now allows ultrasound- trained intensivists to elegantly and noninvasively assess the diaphragm by measuring active diaphragmatic excursion and inspiratory thickening.
Technical Aspects of POCU of the Diaphragm
An ultrasound unit with an M-mode option is needed, also ideally with an anatomic M-mode that allows the operator to steer the scan line to any angle rather than having the line in a strict vertical position. The recommended probe to image diaphragmatic excursion should have a low frequency range (3 to 7 MHz) and the transducer should be either a curvilinear or phase array probe. To measure diaphragmatic thickness, a high frequency (7 to 18 MHz) linear or microconvex probe is preferred. Posture is an important determinant of the breathing pattern: diaphragmatic breathing and excursion is more pronounced in the supine position and is best assessed supine. Normal reference values also differ depending on the position.
Measurement of Diaphragmatic Excursion
Prior to making any measurements, one needs to remove the patient from any positive pressure ventilation, providing it is safe to do so.
The right hemidiaphragm is best imaged through the hepatic window with a low frequency probe. It appears on ultrasound as an easy-to-recognise hyperechogenic (white) line that separates the liver from the lung (see Figure 1A). The left hemidiaphragm is slightly more challenging to visualise given that the splenic window is smaller and more difficult to find than the hepatic window. Two common approaches can be used: anterior subcostal or lateral mid- axillary intercostal. In the anterior approach, the probe is aimed in a dorsal, medial and cephalad direction so that the beam reaches the posterior third of the diaphragm at a perpendicular angle (Boussuges et al. 2009). The beam direction is then adjusted to measure the maximal excursion of the hemidiaphragm. The lateral mid- axillary intercostal approach is often used to assess for pleural effusions and consists of a lateral and longitudinal cut of the hemidiaphragm. Given that the beam is not perpendicular to the diaphragm, the anatomic M- mode is required to adjust the angle accordingly (see Figure 1). The anterior approach is easy on the right side but not always feasible on the left one, due to the interposition of air in the stomach or bowel. Failure to get appropriate images on the left side is around 25% (Scott et al. 2006). For this reason we prefer the lateral approach. If the anatomic M- mode is not available, one can still measure diaphragmatic excursion from still images obtained at the peak of inspiration and expiration. On the first frames at end-expiration and at end- inspiration, a caliper is placed on the leading edge of the diaphragmatic line. The distance separating those two marks corresponds to diaphragmatic excursion.
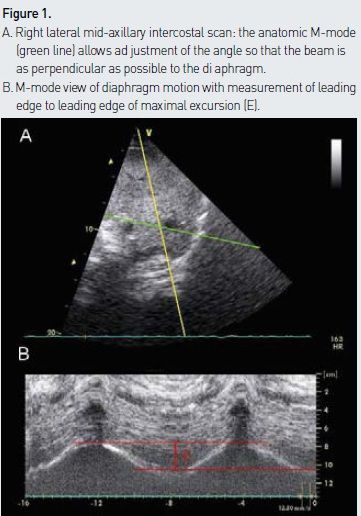
The spontaneously breathing patient is assessed in the supine position to detect striking differences in excursion of the two hemidiaphragms. A cephalad movement during inspiration (paradoxical movement) is consistent with paralysis of one hemidiaphragm or diaphragmatic paralysis if the paradoxical movement is seen on both sides. If no significant asymmetry between the excursions of the two hemidiaphragms is identified, the greatest measured excursion of either hemidiaphragm is recorded for assessing global diaphragmatic function. Boussuges demonstrated that M- mode measurements are reproducible for assessing hemidiaphragmatic movement (Boussuges et al. 2009).
Diaphragmatic Thickening Measurement
A high frequency probe is placed on the midaxillary line at the level of the costophrenic angle. The point of junction between the diaphragm and the chest wall is then identified to measure diaphragmatic thickening. The diaphragmatic muscle is easily identified underneath the intercostal muscles with its characteristic three- layer appearance (a central relatively thick hypoechogenic layer surrounded by two hyperechogenic fine lines corresponding to parietal pleura and peritoneum above and below the diaphragm, respectively) (see Figure 2A). The diaphragmatic muscle is thicker in its lower than its upper portions, and thickening should be measured at the point of maximum thickness using the M-mode (see Figure 2B). Although the measured dimensions are small, the values obtained appear to be reliable and reproducible (Baldwin et al. 2011).
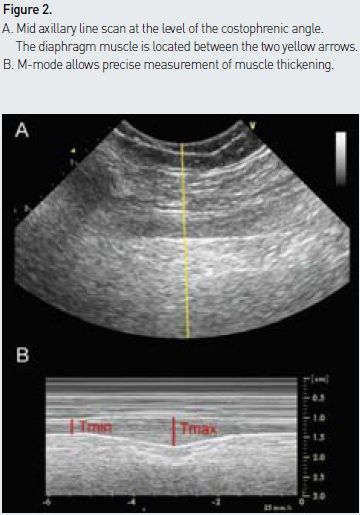
Interpretation and Normal Values
Normal values for diaphragmatic excursion vary in the general population (Gerscovich et al. 2001). As a rule of thumb, the following values are worth remembering: the average excursion of the diaphragm during quiet spontaneous breathing is 20 mm (range: 2- 23 mm) and during deep breathing 60 mm (range: 40-90). Diaphragmatic excursion tends to be greater in males than in females and does not correlate well with age, height or weight. Although maximal excursions often differ between hemidiaphragms, a greater than twofold difference in excursion is to be considered abnormal.
Normal values for diaphragmatic thickening have been published during normal quiet and deep breathing (Harper et al. 2013; Boon et al. 2013). In the general population the average thickness of the diaphragm at its junction with the chest wall (measured at the end of expiration) is 3.0 mm (range, 1.2-12.0) with normal differences between right and left of less than 0.3 mm.
Diaphragmatic thickening may be expressed in two ways: a. thickening ratio (TR = Tmax /Tmin ) or b. thickening index [Δtdi % = (endinspiratory thickness - end- expiratory thickness) / end-expiration thickness]. In healthy subjects, the average TR is 1.2, but sometimes no thickening at all is observed during normal breathing. During a maximal inspiratory effort, the average reported TR is 2 but can vary between 1 and 4. Changes in the thickness of the diaphragm are proportional to its shortening, ie the change in thickness is proportional to the force that the muscle can generate.
Possible Clinical Applications and Words of Caution
When the aetiology of hypercapnic respiratory failure or severe shortness of breath is not fully understood, a neuromuscular cause, including diaphragmatic paralysis, must be considered, and can be assessed using ultrasound as described above. In the ICU, this tool is of great interest for predicting weaning success or failure and understanding the mechanism of failure to wean. Demonstration of diaphragmatic weakness or paralysis is an important clue to the possible cause of weaning difficulty. Ultrasound of the diaphragm also helps predict weaning outcome and compares favourably with traditional indices such as rapid shallow breathing (Jiang et al. 2004). This needs to be confirmed on a larger scale and in less expert hands. Ultrasound of the diaphragm may also be potentially helpful to detect asynchrony between spontaneous diaphragmatic movement and positive pressure ventilation (Matamis et al. 2013).
An important caveat when performing assessment of diaphragmatic excursion is that this requires the patient to be able to be disconnected from the ventilator to remove the confounding passive diaphragmatic movements induced by positive pressure ventilation. The patient must be able to tolerate at least a few minutes of Tpiece and ideally to perform a maximal inspiratory effort on command. Under those conditions, a threshold value of 25 mm can identify patients with significant diaphragmatic dysfunction with good sensitivity and specificity (Lerolle et al. 2009). If patient cooperation cannot be obtained, the measure performed during baseline spontaneous breathing can still provide interesting clues. An excursion of less than 10 mm is usually due to diaphragmatic dysfunction and predictive of a difficult wean (Kim et al. 2011). In another study the average excursion in the patients who failed to wean was 8mm versus 21mm in the successfully weaned group (Giraldo et al. 2011). In obese patients, those measures are more difficult to obtain.
For thickening during maximum inspiration, the average Δtdi % of patients who failed to wean was 16%, versus 55% for those who were successfully weaned (Giraldo et al. 2011). In the study by Dinino et al. (2013) a Δtdi threshold <30% was found to discriminate patients at risk of failing with good sensitivity and specificity.
Thickness of diaphragm at the end of expiration is difficult to interpret and does not predict well strength or endurance. This measurement is highly variable from one individual to another so that the absolute value is of little interest. However, changes over time could indicate the development of diaphragmatic atrophy sometimes seen early after mechanical ventilation initiation. The prognostic value of this atrophy is not yet clear. In contrast, increasing diaphragmatic thickness over time appears to be a favourable prognostic factor for successful weaning from mechanical ventilation (Summerhill 2008).
Conclusion
Diaphragmatic utrasonography is an easy-to-learn and reproducible technique that complements heart and lung ultrasound to pinpoint potential causes for dyspnea, to predict weaning outcome and to assess cause for failure to do so. There is no current adequate substitute for the information provided non-invasively and at low cost by this method. We therefore strongly encourage intensivists to learn this new skill and to incorporate it into their practice. ICU administrators would be wise to budget for ultrasound devices and staff training as meaningful applications of ultrasonography will continue to grow in years to come.
Acknowledgements
Damien Tagan is scientific advisor for CAE Healthcare.





