ICU Management & Practice, Volume 23 - Issue 2, 2023
Extracorporeal organ support (ECOS) involves an extensive use of healthcare resources and clinical problem-solving challenges. The feasibility of applying known ecological analysis and sustainability strategies in healthcare need to be started in this setting.
Introduction
As with any other species, human activity modifies the environment at multiple levels and may or may not alter the ecological balance. As from the industrial revolution, the impact of human activities on the environmental balance increased exponentially; even when more efficient use of natural resources become available, its cost diminishes, increasing its demand as per the Jevons Paradox, leading to increased use of natural resources and continuous waste production, leading to climate change.
The healthcare industry is one of the highest carbon-intensive service sectors representing 4.4–4.% of worldwide greenhouse gas (GHG) emissions and similar fractions of toxic air pollutants (Eckelman et al. 2020; Lenzen et al. 2020).
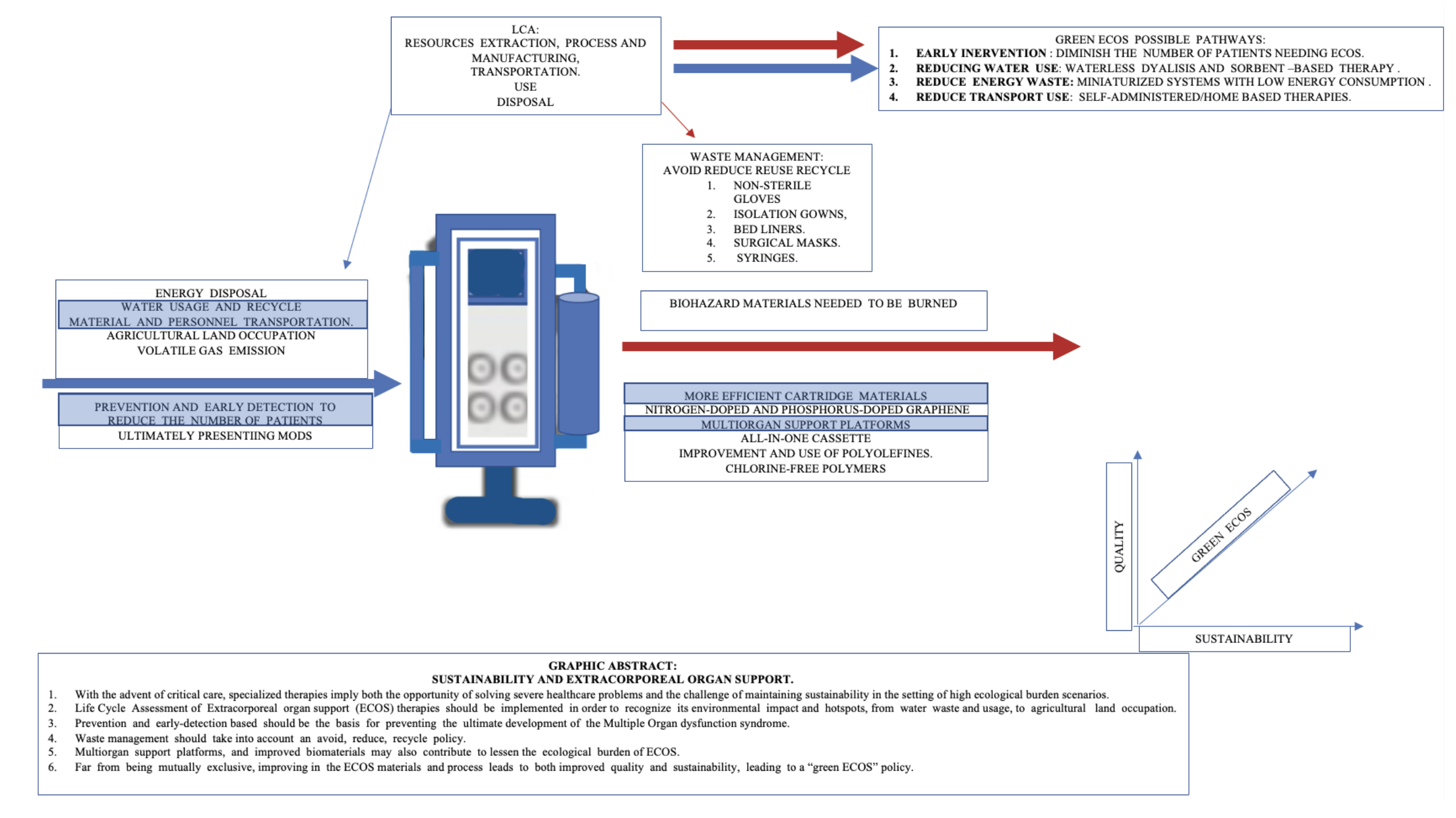
Life cycle assessment (LCA) is a cradle-to-grave assessment that impacts every stage of a product's life cycle, from natural resources extraction, process and manufacturing, transportation, use, and disposal. LCA has been introduced as the definitive method for comparing the ecological footprints of products, processes, and systems (McGain et al. 2020).
In order to improve the sustainability of critical care, LCAs with specific outcomes are compared, such as the carbon footprint of reusable versus single-use devices, allowing to avoid, reduce, reuse, recycle, and reprocess strategies to prospect. Sustainability in healthcare must take into account not only clinical outcomes but also economic, social and environmental costs (McGain et al. 2020).
A significant part of the healthcare-related environmental emissions is indirect or implicit in the manufacturing of products and energy that supports hospitals, so an integrated approach to intensive care sustainability must take into account not only solid waste recycling but also carbon emission reduction and efficient utilisation of natural resources towards a circular economy (McGain et al. 2020).
Carbon Footprint in Critical Care Facilities
Healthcare is carbon intensive. Data from the National Health Service in the United Kingdom indicates that two-thirds of GHG emissions arise from purchasing consumables and one-third directly from hospital energy use and transport. Among healthcare facilities, intensive care units (ICU) considerably consume items and generate waste.
A critically ill patient with septic shock has a daily carbon footprint that equates to 3.5 times that of a healthy individual in the U.S. (McGain et al. 2018; Baid and Damm 2021).
McGain et al. (2018), in a prospective, observational LCA that included the use of energy (for heating, ventilation, air conditioning, lighting, machines), consumables and waste involved in the ICU treatment of septic shock patients, described that the average energy use per patient could reach 272 kWh/day, while the average use of single-use materials per patient per day could reach 8.7 kg. Daily GHG emissions expressed in carbon dioxide equivalent (CO2-e) could reach 228 kg CO2-e, with natural gas being the most important source of GHG emissions. This data showed that carbon footprint was mainly due to the use of energy for heating, ventilation and air conditioning, with consumables being less important.
The ECOS Definition and Healthcare Needs Implications
In critically ill patients with severe forms of single or multiple organ dysfunction, pharmacological and/or surgical treatment may not be enough. In these complex cases, a single form of extracorporeal organ support (ECOS) or even multiple organ support therapy (MOST) may be needed and is increasingly seen as a feasible approach (Ronco et al. 2019).
Considering the complex crosstalk mechanism between native organs, it is not surprising that patients with one organ failure may develop multiple organ dysfunction syndrome (MODS) later in the clinical course, thus needing multiple organ extracorporeal support, either by combined or integrated ECOS devices (Ronco et al. 2019; Huber et al. 2020).
MODS is one of the most common causes of death in ICU patients. Based on extensive and long-term use of renal replacement therapy (RRT), ECOS became available for other organ failures. In the beginning, these techniques, including RRT, extracorporeal membrane oxygenation (ECMO), extracorporeal CO2 removal (ECCO2R) and extracorporeal liver support, were used as stand-alone single-organ support systems (Ronco et al. 2019; Huber et al. 2020).
The concept of MOST and giving simultaneous and combined support for different failing organs was described more than 15 years ago by Ronco and Bellomo. This concept implicates the advent of multidisciplinary and multiprofessional strategies in the treatment and improvement of MODS patients in the ICU (Ronco et al. 2019; Huber et al. 2020).
Even when there are no specific LCA studies for patients with MODS under MOST, several studies have investigated the ecological burden and plausible strategies to improve the ecological (and human) burden cost/benefit ratio (Huber et al. 2020). First of all, material flow should be taken into account, as it is common to all of the ECOS systems in the ICU. A material flow analysis (MFA) in an academic ICU showed a material mass inflow of 247,000 kg per year, of which 50,000 kg was incinerated as hazardous hospital waste. The environmental impact per patient resulted in 17 kg of mass, 12 kg CO2 eq, 300L of water usage and 4 square metres of agricultural land occupation per day, with five identified hotspots: non-sterile gloves, isolation gowns, bed liners, surgical masks and syringes (Hunfeld et al. 2023; Barraclough and McAlister 2022).
As many patients needing ECOS will need surgical procedures or may use anaesthetic gases as a part of the sedation strategy during ventilatory support, the ecological burden of anaesthetic gases should be taken into account (Soreze et al. 2020; Fabien et al. 2022; Bellgardt et al. 2021; Bomberg et al. 2016; Romagnoli et al. 2017; Herzog-Niesery et al. 2019).
Even when climate change was initially postulated by Fourier in the 1820s, it was in the 1970s that real concerns emerged, with the majority of increases occurring after 1980. Since the 1960s, the effects of other increasing GHGs (most of all CH4, N2O, O3, and halogenated compounds) contribute as much to global warming as increasing CO2 itself, with halogenated compounds (including volatile anaesthetic agents) accounting for approximately 11%. Nitrous oxide is responsible for the majority of ongoing ozone depletion and approximately 6% of anthropogenic global warming (McGain et al. 2020; Barraclough and McAlister 2022).
All heteronuclear gases, as well as some limited homonuclear molecules, vibrate/rotate/stretch in the presence of infrared radiation (infrared active). Absorption and subsequent emission of infrared light reduces the heat radiation from Earth to space (also called heat retention), described by the term global warming potential (GWP). Carbon dioxide has, by definition, a GWP of 1, while N2O has a GWP of 265 (McGain et al. 2020; Barraclough and McAlister 2022).
Solar radiation enters the atmosphere, and infrared radiation exits as heat. If more radiation is entering Earth than leaving, it is called radiative forcing. Halogenated anaesthetic ethers, isoflurane, enflurane, and desflurane have similar radiative forcings; while sevoflurane has about 25% less radiative forcing. Halothane, not having the great infrared absorption of an ether group, has about half of sevoflurane's radiative force (McGain et al. 2020; Barraclough and McAlister 2022).
Expressing these CO2e emissions as equivalent distance driven, one MAC-hour (2.2% sevoflurane, 1.2% isoflurane, 6.6% desflurane, at 1L min−1 fresh gas flow), sevoflurane is equivalent to 6.5 km, isoflurane to 13 km, and desflurane 300 km (McGain et al. 2020). The most important, safe, and effective measures to reduce carbon related to anaesthesia are to avoid desflurane and N2O, practice low-flow anaesthesia, and minimise the use of inhalation agents by using regional and/or total intravenous anaesthesia (TIVA).
Even when there are no large studies on specific ECMO, albumin dialysis or haemadsorption carbon footprint, haemodialysis may be considered a starting comparison point in this model as various initiatives, including the green dialysis initiative, are aimed to address environmental sustainability regarding RRT, and some strategies and algorithms may serve as a template for developing those aimed at other forms of ECOS (Gauly et al. 2022; Barraclough and Agar 2020).
Considered emissions taken into account in the already described studies on RRT carbon footprint have included electricity, natural gas, water, and supply use; patient and staff travel distance, as well as biohazard and landfill waste emission (Sehgal et al. 2022; Barraclough and McAlister 2022).
A study of LCA of GHG emissions in carbon dioxide equivalents (CO2-eq) associated with 209,481 haemodialysis treatments in the year 2020 reported that annual emissions per facility averaged 769,374 kg CO2-eq, being the largest contributors to total emissions - patient and staff transportation (28.3%), electricity (27.4%), and natural gas (15.2%) (Gauly et al. 2022; Sehgal et al. 2022).
Each treatment equated to 58.9 kg CO2-eq, with a three-fold variation across facilities, being the contributors with the largest variation in transportation, natural gas, and water. The annual emissions per haemodialysis facility equates to those in 93 homes; emissions per treatment are equivalent to driving an average automobile for 238 km. Over 500L of water, 7 kW of energy and approximately one kilogram of medical waste are consumed during haemodialysis (Gauly et al. 2022; Sehgal et al. 2022; Wieliczko et al. 2020).
Also, it should be taken into account that the production of 1L of ultrapure water for dialysis requires 1.5–1.7 raw water (which means 60–70% water), but it is still portable and can be used for cleaning, washing or gardening, saving at least 100,000,000m3 annually (Wieliczko et al. 2020).
Regarding diminishing material waste by improving the efficacy and quality of the platform used in a new HD system, the conventional blood line system (and in the case of on-line haemodiafiltration, additionally a substitution line) is replaced by an all-in-one cassette system unifying all the components of the extracorporeal circuit, diminishing the total disposable weight and simplifying the operation of the HD system (Wieliczko et al. 2020).
Through cassette design improvement and the use of polyolefins, unused disposable is reduced by 100g in comparison to bloodlines used for other HD systems. For a centre performing 10,000 treatments annually, this leads to hazardous waste reduction by approximately 1500–2000 kg. Another alternative to reduce waste by design is performing on-line priming and rinsing in the set-up phase, as well as on-line infusions and reinfusion at the end of the session both in HD and on-line HDF instead of applying saline from an extra bag (Wieliczko et al. 2020).
Waste composition is also relevant to ensure the safe management of healthcare waste, as it is separated into infectious and non-infectious for incineration or landfill and recycling, respectively. For components that will be incinerated (including the extracorporeal system in HD), it is desirable that polyvinyl chloride (PVC) could be replaced by chlorine-free polymers in order to minimise the formation of dioxins and furans, which are generated at insufficiently high temperatures (Wieliczko et al. 2020; Barraclough and Agar 2020).
Another way of improving the sustainability of ECOS is improving the haemofilter, as per the functionality and footprint of the device: improving the quality of ECOS implies not only a water-saving strategy but may also diminish the waste of biohazardous materials:
Adding a biocompatible polymer coating agent to the haemofilters may lower the incidence of thrombosis of the haemofilter, diminishing the number of haemofilters ultimately being used (Tagaya et al. 2019).
Extracorporeal blood purification can be achieved by diffusion (as in standard haemodialysis), convection (as in haemofiltration), diffusion and convection (as in haemodiafiltration) or by solute adsorption, based on mass separation by a solid agent (sorbent). Haemoperfusion may also be used in combination with haemodialysis, haemofiltration and even haemodiafiltration, allowing for toxic solute removal from the blood with lesser use of water. Clinical uses of sorbents in sepsis, acute kidney injury, and liver diseases have so far provided data on their feasibility and safety (Ronco and Bellomo 2022).
Sorbent cartridge design should consider multiple aspects, including the cost of the polymers, high resistance to fouling, maximal biocompatibility, and the absence of undesirable side effects. The porosity, polymers, and internal pathways within the cartridge should maximise the mass transfer along the sorbent bed, along with the prospected flow rate (Ricci et al. 2022).
Within the critical care scenarios, sorbents have proved to improve clinical outcomes, including diminishing hospital length of stay in multiple clinical scenarios, including ECMO, sepsis, liver failure, rhabdomyolysis and intoxication (Ricci et al. 2022).
The use of a polymeric sorbent based on phenylglyoxaldehyde, that covalently binds urea under physiological conditions has been described as a sorbent-based strategy for urea removal as a step towards the wearable artificial kidney (Jong et al. 2020).
Also, molecular dynamic simulation of urea removal on carbon nanosheets has been reported using nitrogen-doped and phosphorus-doped graphene. The results further offer attractive suggestions for novel adsorbents for artificial kidney devices and the development of novel and enhanced urea adsorbents (Karimi and Rahsepar 2022).
In addition, haemoperfusion with a neutral microporous resin column in patients with extrapulmonary sepsis-induced acute lung injury has reported removal of plasma and bronchoalveolar lavage TNF-α and IL-1, improvement of PaO2/FiO2, as well as radiological improvement (Huang et al. 2013).
By reducing both water use and hospital length of stay, haemoperfusion techniques may ultimately reduce the total carbon footprint of a single MODS-related stay in ICU patients. Even when carbon footprint-oriented comparison clinical trials are yet to be developed, sorbent technology may represent a huge contribution to an environmentally friendly ECOS.
Conclusion and Perspectives
Time is pressing, and critical care medicine must participate in the race to zero-emission healthcare systems. ECOS represents both clinical and ecological challenges, as it implies the challenge of solving severe healthcare problems while maintaining sustainability in high ecological burden scenarios (Bein et al. 2021). In order to improve the sustainability of ECOS, LCA of specific analysis should be done, while the feasibility of strategies already described in the setting of other hospital facilities and clinical scenarios, including RRT, should be considered for other ECOS scenarios (Baid and Damm 2021).
So far diffusion and convection-based extracorporeal therapies that require energy and water consumption have been used. New miniaturised systems with battery-operated pumps, low energy consumption and waterless dialysis technologies based on sorbents are probably interesting pathways to undertake in order to reduce the ecological impact of ECOS on the ICU, and also to possibly provide the basis for self-administered or even home-based therapies. Clinical trials focused on both improving the efficacy and sustainability of ECOS are yet to come, as critical care poses an utmost responsibility for contributing to the health system sustainability challenge.
Conflict of Interest
None.
References:
Baid H, Damm E. (2021) Reducing critical care’s carbon footprint with financial and social co-benefits. Intensive & Critical Care Nursing. 64:103030.
Barraclough KA, McAlister S (2022) Assessing the carbon footprint of hemodialysis: A first step toward environmentally sustainable kidney care: A first step toward environmentally sustainable kidney care. Journal of the American Society of Nephrology. 33(9):1635-1637.
Barraclough KA, Agar JWM (2020) Green nephrology. Nature reviews. Nephrology. 16(5):257-268.
Bein T, Koch S, Schulz C (2021) What’s new in intensive care: environmental sustainability. Intensive Care Medicine. 47(8):903–905.
Bellgardt M et al. (2021) Extracorporeal membrane oxygenation and inhaled sedation in coronavirus disease 2019-related acute respiratory distress syndrome. World journal of critical care medicine, 10(6):323-333.
Bomberg H et al.. (2016) AnaConDaTM and MirusTM for intensive care sedation, 24 h desflurane versus isoflurane in one patient. SpringerPlus, 5(1):420.
Eckelman MJ et al. (2020) Healthcare pollution and public health damage in the United States: An update. Health Affairs. 39(12):2071–2079.
Fabien J et al. (2022) Inhaled isoflurane sedation through the AnaConDa anesthetic conserving device in prehospital emergencies. Emergencias. 34(6):481-482.
Gauly A, Fleck N, Kircelli F (2022) Advanced hemodialysis equipment for more eco-friendly dialysis. International Urology and Nephrology. 54(5):1059-1065.
Herzog-Niescery J et al. (2019) Environmental safety: Air pollution while using MIRUSTM for short-term sedation in the ICU. Acta Anaesthesiologica Scandinavica. 63(1):86-92.
Huber W, Ruiz de Garibay AP (2020) Options in extracorporeal support of multiple organ failure. Medizinische Klinik, Intensivmedizin und Notfallmedizin. 115(Suppl 1):28–36.
Hunfeld N et al. (2023) Circular material flow in the intensive care unit-environmental effects and identification of hotspots. Intensive Care Medicine. 49(1):65–74.
Karimi K, Rahsepar M (2022) Optimization of the urea removal in a wearable dialysis device using nitrogen-doped and phosphorus-doped graphene. ACS Omega. 7(5):4083-4094.
Lenzen, M et al. (2020) The environmental footprint of health care: a global assessment. The Lancet Planetary Health. 4(7):e271–e279.
McGain F et al. (2020) Environmental sustainability in anaesthesia and critical care,” British Journal of Anaesthesia.125(5):680-692.
McGain F et al. (2018) The carbon footprint of treating patients with septic shock in the intensive care unit. Critical Care and Resuscitation. 20(4):304-312.
Romagnoli S et al. (2017) The new MIRUS system for short-term sedation in postsurgical ICU patients. Critical Care Medicine. 45(9):e925–e931.
Ronco C, Ricci Z, Husain-Syed F (2019) From multiple organ support therapy to extracorporeal organ support in critically ill patients. Blood purification. 48(2):99-105.
Sehgal AR, Slutzman JE, Huml AM (2022) Sources of variation in the carbon footprint of hemodialysis treatment. Journal of the American Society of Nephrology. 33(9):1790-1795.
Soreze Y et al. (2020) Sevoflurane sedation with AnaConDa-S device for a Child Undergoing Extracorporeal Membrane Oxygenation,” Indian Journal of Critical Care Medicine. 24(7):596-598.
Wieliczko M et al. (2020) Eco-dialysis: fashion or necessity. International urology and nephrology. 52(3):519-523.








