ICU Management & Practice, Volume 23 - Issue 2, 2023
The timing and application of dialysis in the ICU is highly variable contributing to poor outcomes. A clinical decision support system (CDSS) incorporating a dynamic predictive algorithm for organ support could improve outcomes.
Background
Multiorgan failure (MOF) and acute kidney injury (AKI) are frequently encountered in critically ill patients and often require acute dialysis for support to facilitate recovery. There is considerable variation in the application of dialysis that is associated with mortality rates >50% and high resource utilisation (Aglae et al. 2019; Ethgen et al. 2015; Silver et al. 2017; Dasta and Kane-Gill 2019; Gaudry et al. 2020; Harding et al. 2020; Ethgen et al. 2022). Decisions for acute dialytic intervention require consideration of who would benefit from dialysis and when would it be best to intervene (Macedo and Mehta 2017). Current guidelines for the timing of dialysis are often disparate and rely largely on the presence of specific indications to identify who needs dialysis with the urgency of intervention depending on the presence of life-threatening complications (NICE guidelines 2019; Bouchard and Mehta 2022). This approach makes it difficult to identify and track high risk patients before they meet an indication and to define the optimal time point for intervention based solely on the presence or absence of the indication. The underlying severity of kidney dysfunction (Stage of AKI, oliguria) is viewed as the major driver for dialytic intervention and was the primary inclusion criteria for all the trials (Gaudry et al. 2016; Zarbock et al. 2016; Barbar et al. 2018; Investigators Canadian Critical Care Trials Group et al. 2020). However, not all AKI stage 3 patients require dialysis and it is often started in the absence of AKI for fluid management or adjunctive organ support and AKI (Ostermann et al. 2016).
Recent trials have shown application of dialysis in the ICU is highly variable and based on current criteria results in over 40% of patients not needing dialysis while those who receive it late have a 25% higher mortality (Bouchard and Mehta 2022). In the largest of these trials (STAART AKI) clinician equipoise was utilised to determine enrolment of patients who met a Stage 2 AKI, almost one third of patients were enrolled where the physician was uncertain of the benefit of dialysis (Investigators Canadian Critical Care Trials et al. 2020) An analysis of the patients screened and not enrolled in the STAART trial, showed that clinicians excluded over 89% of provisionally eligible patients due to their personal equipoise, however 8.7% of patients who were mandated immediate RRT were not dialysed and 11% who were considered to not require dialysis received it (Wald et al. 2021). In the latest AKIKI 2 trial comparing the effect of deferring dialysis for severe AKI until there was evidence of severe complications there was higher risk of mortality at 60 days despite similar rates of complications (Investigators Canadian Critical Care Trials et al. 2020). In a post hoc analysis risk stratification profiles of patients dialysed within 48 hrs. of randomisation to a delayed strategy, showed that patients in the fourth and fifth quintiles of risk would have benefitted from an early intervention demonstrating considerable heterogeneity of treatment effect of early vs delayed dialysis (Grolleau et al. 2022). These clinical trials have led to considerable controversy on whether a “wait and see approach” is preferable to an early intervention (Zarbock and Mehta 2019; Bouchard and Mehta 2020; Meraz-Munoz et al. 2021; Pan et al. 2021; Gaudry et al. 2022). However, there is no clarity on which patients would benefit from waiting, the parameters that should define the waiting period and its duration and how patients should be managed during the waiting period (Bagshaw et al. 2021). The recent COVID-19 pandemic created an unprecedented strain on healthcare resource utilisation, as there was a marked increase in patients requiring acute dialysis that overwhelmed available resources in many centres (Chan et al. 2021; Gupta et al. 2021). These experiences highlight the need for standardised comprehensive systems-based approaches for enabling providers and healthcare systems to identify patients who would most benefit from high acuity care while de-escalating care for others (Stevens et al. 2021; Rhee et al. 2022).
The Role of Predictive Analytics
Current lack of accepted standards for timing of dialysis initiation, heterogeneity of patients and variations in care delivery contribute to under and over utilisation of the therapy resulting in high mortality, increased length of stay, rehospitalisation and long-term need for dialysis at a cost of over 10 billion dollars per year in the U.S. Consequently, there is a great need for tools to identify high risk patients who would need dialysis, determine the optimal time for intervention with the best likelihood for benefit while minimising risk and to adjust the operational characteristics to personalise management. While current scoring systems (e.g., APACHE 3, SOFA) provide an objective measure of patient condition and organ dysfunction, they do not support decision making in effective resource deployment of extracorporeal organ support (ECOS) (Aziz et al. 2020). The availability of real time data in electronic health records has led to the development of several machine learning models predicting the development of AKI and subsequently the requirement of dialysis. These models incorporate several variables; however, lack transparency of how the components in the model interact limits interpretability for clinical care and transferability across centres (Koyner et al. 2018; Tomasev et al. 2019; Churpek et al. 2020; Goldstein and Bedoya 2020; Vaid et al. 2021). Biomarker-based approaches have similarly been assessed to predict the provision of dialysis, but no single biomarker has emerged as being predictive (Klein et al. 2018; Fiorentino et al. 2020). The lack of utility for these techniques represents prior approaches that create risk predictions based on assessment of single points in time and do not incorporate changes in patient course and do not utilise available clinical and lab data for dynamic risk assessment and intervention (Wilson et al. 2021; Demirjian et al. 2022).
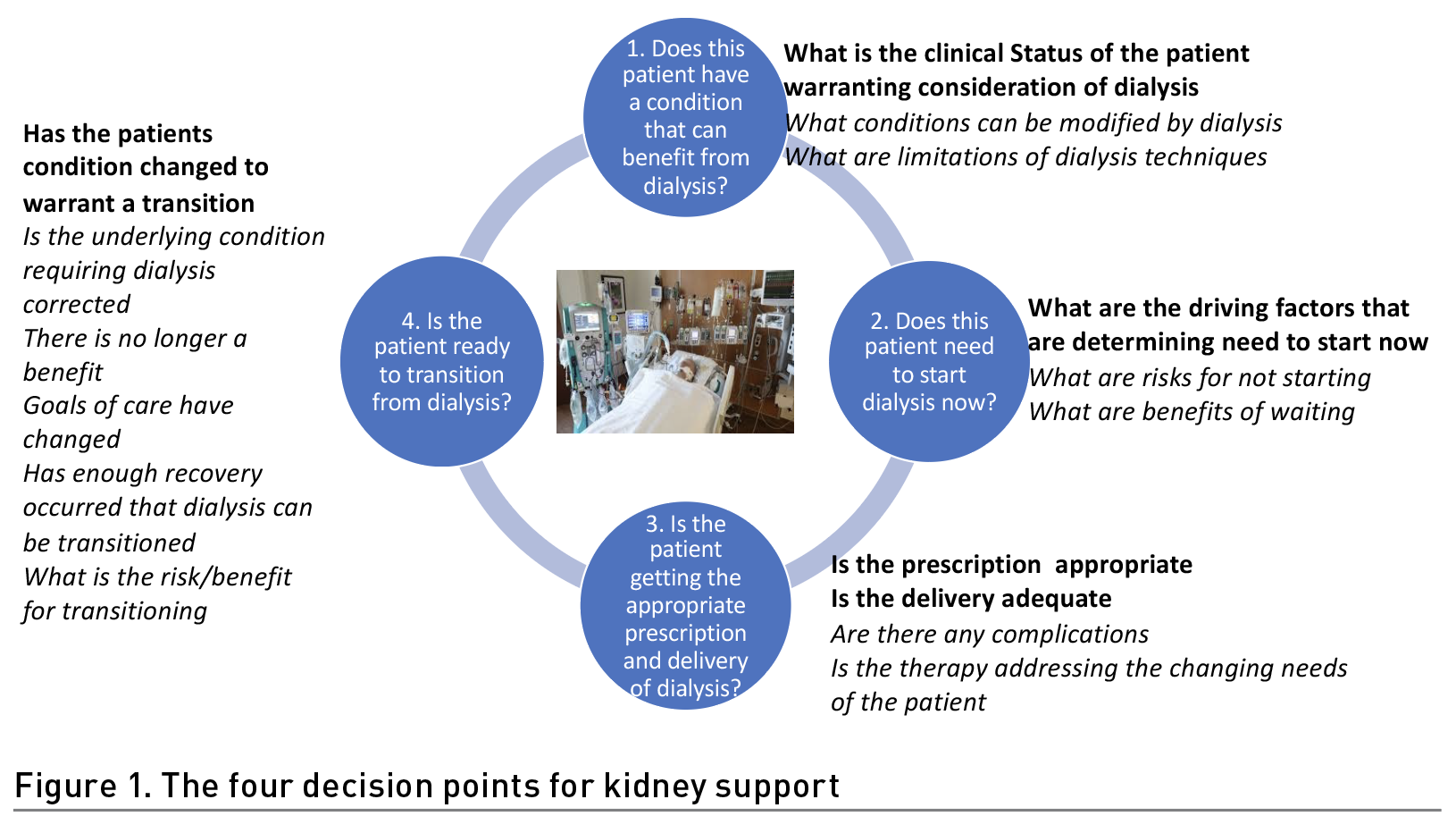
It is instructive to consider the usefulness of prediction is to assist clinicians in making decisions for diagnostic or therapeutic interventions. When dialysis is considered in critically ill patients, there are four decision points (Figure 1). Assessing the clinical status of the patient and evaluating the potential benefit and risks of intervention are an essential first step where knowledge of the operational characteristics of the therapy (modality, dose, duration) need to be matched with the clinical condition. Once dialysis is determined to be appropriate, defining the optimal time for intervention is paramount to avoid unnecessary treatments and delays that deprive the patient of achievable benefit and may contribute to futility. Deciding the modality, dose, and management strategies to adjust therapy delivery to meet the changing needs of the patients and determine transitions in therapy to match goals of care are additional decision points that are required. There is wide discrepancy in these decision points that lead to variations in care delivery and could be amenable to improvement with predictive analytics. In most cases clinicians make decisions for dialysis support based on an assessment of the accessible clinical and lab data, evaluation of the patient’s condition and the anticipated course. However, patients span the spectrum of presentation ranging from a relatively stable course to a rapid progression with multiorgan failure. To improve decision making and timely interventions with dialysis it is crucial for physicians to evaluate and integrate disparate pieces of information that are often not concurrently available. Clinical decision support systems (CDSS) can reduce the burden by continuously collecting relevant data within electronic health records (EHR), integrating the information and presenting it to clinicians for timely action (James et al. 2022).
Development of a CDSS for Kidney Support in the ICU
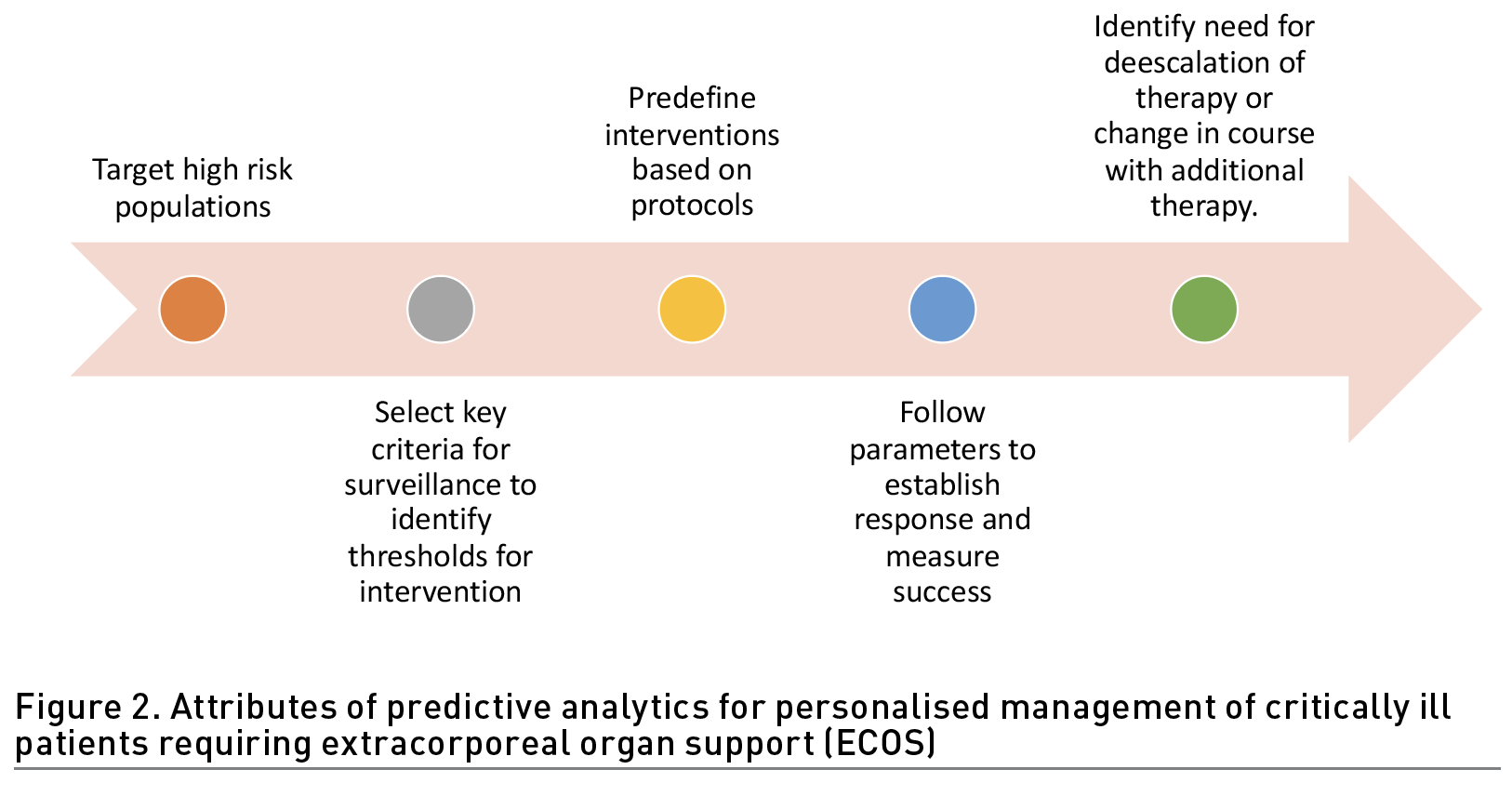
Recent studies have provided evidence for the potential benefit of real-time high-resolution assessments every few minutes, to provide an earlier indication of developing sepsis and organ failure (Adams et al. 2022; Henry et al. 2022). Nevertheless, prediction by itself is not enough as it should provide actionable information leading to specific interventions that influence the course favourably. As shown in Figure 2 predictive analytics applied for informing application of organ support should encompass dynamic assessments in a continuum of care. Identifying high risk patients at ICU admission should lead to active surveillance for thresholds for intervention, guide the choice of dialysis modality and its delivery to address the clinical need and provide measures to monitor the course with appropriate changes in therapy to promote recovery.
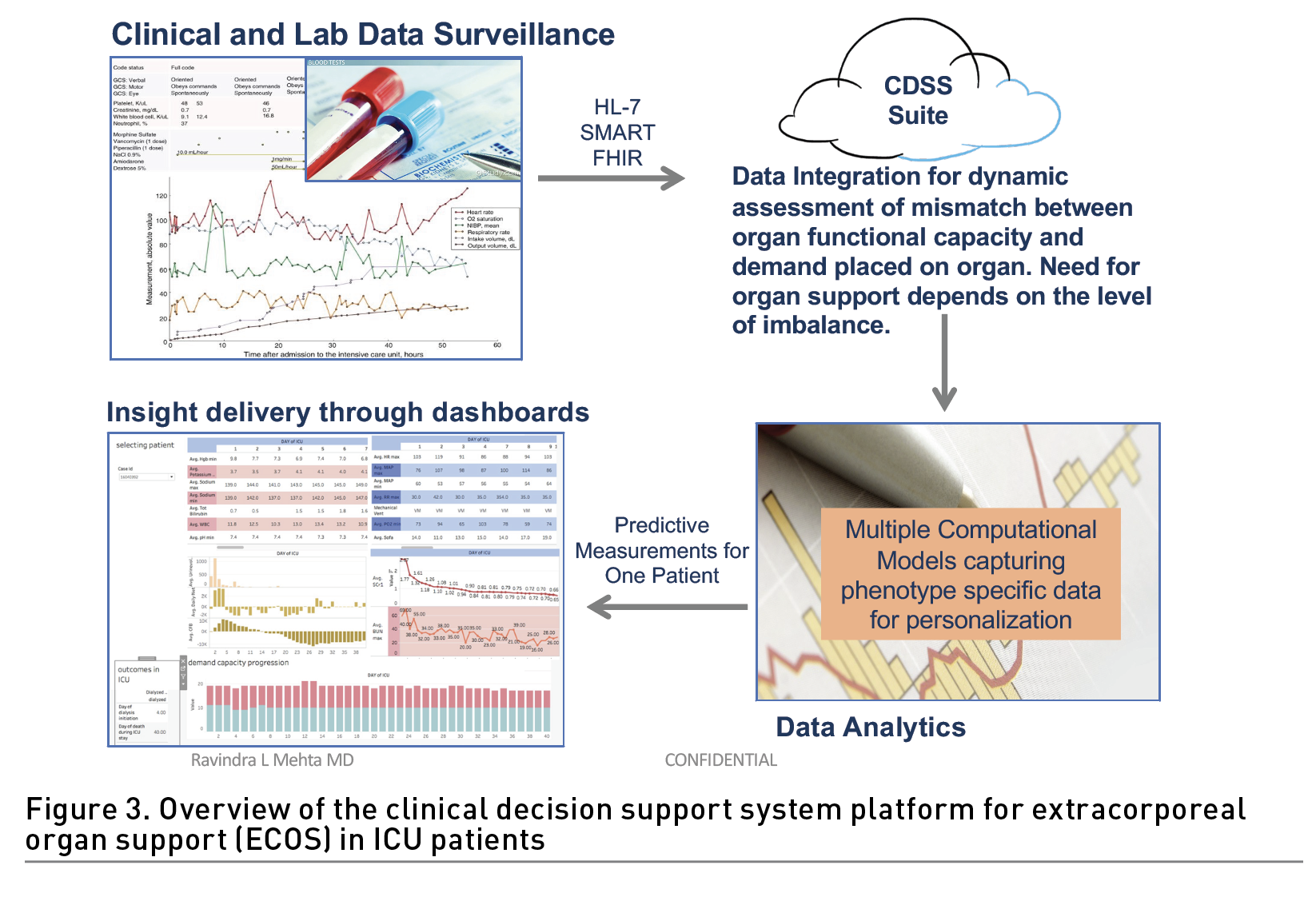
To address these challenges, we have developed a clinical decision support (CDSS) platform for real-time and hospital-wide prediction of acute dialysis requirement. The system relies on a novel method of organ monitoring that assesses the organ’s functional capacity and, using only 20 commonly available patient data-points, predicts when capacity is exceeded to the point that dialytic intervention is required. The system dynamically quantifies the need for organ support using a patent pending Demand/Capacity/Mismatch algorithm (DCM) to assess the mismatch (M) of the demand (D) placed on any organ and the available organ capacity (C) (Ostermann et al. 2016). The DCM utilises patient demographics, clinical characteristics and laboratory data routinely recorded as standard of care in ICU’s worldwide to dynamically quantify individual parameters contributing to the demand and residual capacity of each organ and the additional capacity provided by the dialysis system to determine the response to treatment. Using data from over 20,000 patients throughout the United States, Canada and Europe to develop the algorithms with external validation in an independent set of electronic medical record data from 80,000 patients, this algorithm demonstrated an AUC of 0.945 predicting the need for dialysis within 96 hours at any time in the ICU. Our system has a cloud-based platform constructed in the Amazon Web Services (AWS) environment that links to electronic health records through Fast Healthcare Interoperability Resources (FHIR) and HL-7 endpoints to securely obtain real time data from multiple patient records; parse it accurately through the algorithm whenever new data is available or at defined intervals, to provide clinicians with actionable information in a customisable dashboard that can be configured to alert clinicians at set thresholds (Figure 3). The platform has built in features that support its use across different EHRs and settings and data results can be configured for visualisation in multiple formats.
Conclusions
We have developed and validated a proprietary patent pending, real-time, scalable clinical decision support system (CDSS) to accurately predict risk of organ failure and outcomes in critically ill patients and empower clinicians to achieve timely decisions and implement appropriate organ support at the right time. Our approach to combine real-time predictive risk assessment with prescriptive analytics provides timely, standardised, and optimised clinical decision support through the continuum of a patient’s course of disease. We anticipate its deployment and validation in prospective clinical trials and its utilisation to improve care of critically ill patients.
Acknowledgment
This work was supported through NIH NIDDK Grant DK079337 for the UAB-UCSD O’Brien Center for AKI Research.
Conflict of Interest
Dr Mehta has consulting agreements with Baxter, AM Pharma, Biomerieux, Mallinckrodt, GE Healthcare; Sanofi; Abiomed; NovaBiomed; Novartis; Renasym; Fresenius; and Guard. He has also received grant support from Fresenius; Fresenius-Kabi.
References:
Adams R et al. (2022) Prospective, multi-site study of patient outcomes after implementation of the TREWS machine learning-based early warning system for sepsis. Nat Med. 28(7):1455-1460.
Aglae C et al. (2019) Heterogeneity of Cause, Care, and Prognosis in Severe Acute Kidney Injury in Hospitalized Patients: A Prospective Observational Study. Can J Kidney Health Dis. 6: 2054358119892174.
Aziz S et al. (2020) Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med.
Bagshaw SM et al. (2021) When should we start renal-replacement therapy in critically ill patients with acute kidney injury: do we finally have the answer? Comment. Critical Care. 25(1).
Barbar SD et al. (2018) Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med. 379(15):1431-1442.
Bouchard J, Mehta RL (2020) Wait and see approach for dialysis in acute kidney injury. Nat Rev Nephrol. 16(12):707-708.
Bouchard J, Mehta RL (2022) Timing of Kidney Support Therapy in Acute Kidney Injury: What Are We Waiting For? Am J Kidney Dis. 79(3):417-426.
Chan L et al. (2021). AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 32(1):151-160.
Churpek MM et al. (2020). Internal and External Validation of a Machine Learning Risk Score for Acute Kidney Injury. JAMA Netw Open. 3(8):e2012892.
Dasta JF and Kane-Gill S (2019) Review of the Literature on the Costs Associated With Acute Kidney Injury. J Pharm Pract. 32(3): 292-302.
Demirjian S et al. (2022) Predictive Accuracy of a Perioperative Laboratory Test-Based Prediction Model for Moderate to Severe Acute Kidney Injury After Cardiac Surgery. JAMA. 327(10):956-964.
Ethgen O et al. (2015) Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients. Nephrol Dial Transplant. 30(1):54-61.
Ethgen O et al. (2022) Early versus delayed initiation of renal replacement therapy in cardiac surgery associated acute kidney injury: an economic perspective. J Crit Care. 69: 153977.
Fiorentino M et al. (2020) Serial Measurement of Cell-Cycle Arrest Biomarkers [TIMP-2] . [IGFBP7] and Risk for Progression to Death, Dialysis, or Severe Acute Kidney Injury in Patients with Septic Shock. Am J Respir Crit Care Med. 202(9):1262-1270.
Gaudry S et al. (2020) Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 395(10235):1506-1515.
Gaudry S et al. (2016). Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 375(2):122-133.
Gaudry S et al. (2022) Extracorporeal Kidney-Replacement Therapy for Acute Kidney Injury." N Engl J Med. 386(10):964-975.
Goldstein BA, Bedoya AD (2020) Guiding Clinical Decisions Through Predictive Risk Rules. JAMA Network Open. 3(8).
Grolleau F et al. (2022) Personalization of renal replacement therapy initiation: a secondary analysis of the AKIKI and IDEAL-ICU trials. Crit Care. 26(1):64.
Gupta S et al. (2021). AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J Am Soc Nephrol. 32(1):161-176.
Harding JL et al. (2020) US Trends in Hospitalizations for Dialysis-Requiring Acute Kidney Injury in People With Versus Without Diabetes. Am J Kidney Dis. 75(6):897-907.
Henry KE et al. (2022) Factors driving provider adoption of the TREWS machine learning-based early warning system and its effects on sepsis treatment timing. Nat Med. 28(7):1447-1454.
James MT et al. (2022) Effect of Clinical Decision Support With Audit and Feedback on Prevention of Acute Kidney Injury in Patients Undergoing Coronary Angiography: A Randomized Clinical Trial. JAMA 328(9):839-849.
Klein SJ et al. (2018) Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 44(3):323-336.
Koyner JL et al. (2018). The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit Care Med. 46(7):1070-1077.
Macedo E, Mehta RL (2017) Guiding Physician Decisions for Initiating Dialysis for AKI: Is Progress on the Horizon? Clinical Journal of the American Society of Nephrology. 12(2):217-219.
Meraz-Munoz AY et al. (2021) Timing of kidney replacement therapy initiation in acute kidney injury." Curr Opin Nephrol Hypertens. 30(3):332-338.
National Institute for Health and Care Excellence (NICE) (2019) Acute kidney injury: prevention, detection and management. Available at www.ncbi.nlm.nih.gov/books/NBK552160/
Ostermann M et al. (2016) Patient Selection and Timing of Continuous Renal Replacement Therapy. Blood Purif. 42(3):224-237.
Pan HC et al. (2021) Accelerated versus standard initiation of renal replacement therapy for critically ill patients with acute kidney injury: a systematic review and meta-analysis of RCT studies. Crit Care. 25(1):5.
Rhee H et al. (2022) Maintenance of the critical care system during the pandemic in non-COVID-19 patients requiring continuous renal replacement therapy: a single center experience. BMC Emerg Med. 22(1):138.
Silver SA et al. (2017) Cost of Acute Kidney Injury in Hospitalized Patients. J Hosp Med. 12(2):70-76.
Stevens JS et al. (2021) Continuous renal replacement therapy and the COVID pandemic. Semin Dial. 34(6):561-566.
The STARRT-AKI Investigators for the Canadian Critical Care Trials Group, the Australian and New Zealand Intensive Care Society Clinical Trials Group, the United Kingdom Critical Care Research Group, the Canadian Nephrology Trials Network, and the Irish Critical Care Trials Group Investigators (2020). Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 383(3): 240-251.
Tomasev N et al. (2019) A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 572(7767): 116-119.
Vaid A et al. (2021) Predictive Approaches for Acute Dialysis Requirement and Death in COVID-19. Clin J Am Soc Nephrol. 16(8):1158-1168.
Wald R, Bagshaw SM; STARRT-AKI Investigators (2021) Integration of Equipoise into Eligibility Criteria in the STARRT-AKI Trial. American Journal of Respiratory and Critical Care Medicine. 204(2):234-237.
Wilson TA et al. (2021) Derivation and External Validation of a Risk Index for Predicting Acute Kidney Injury Requiring Kidney Replacement Therapy After Noncardiac Surgery. JAMA Netw Open. 4(8):e2121901.
Zarbock A et al. (2016) Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 315(20):2190-2199.
Zarbock A, Mehta RL (2019) Timing of Kidney Replacement Therapy in Acute Kidney Injury. Clin J Am Soc Nephrol. 14(1):147-149.