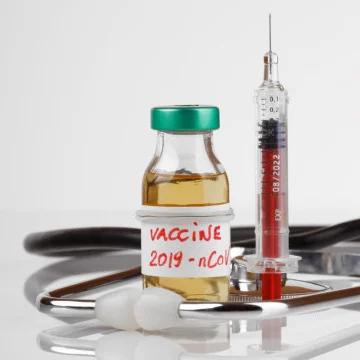
The UK, first in the world, authorises Pfizer COVID-19 vaccine and is looking into fast-tracking AstraZeneca’s. In the meantime, Pfizer and Moderna both file applications for their vaccines’ emergency use in the U.S. and Europe.
You might also like:Coronavirus, Tensegrity and CSR: Year of Living Dangerously
The Pfizer/BioNTech coronavirus vaccine has been approved for use by the MHRA, the U.K. medicines regulator – the first such move globally.
The first 800,000 doses may become available in Britain in the next few days to those at the top of the priority list, i.e. elderly people in care homes and care home staff as well as those over 80 and health and care staff. Overall, the UK has ordered 40m doses of the vaccine, which is produced at Pfizer’s facilities in Belgium.
Since the vaccine requires specific storage conditions (it must be kept at -70C), it will be transported in special boxes of up to 5,000 doses, packed in dry ice. Also, most likely the first vaccinations will be taking place at hospitals where such storage facilities are available. This means that care home staff, NHS staff and patients will be the first to be inoculated. The vaccine will be free and the vaccination will not be compulsory.
The Pfizer/BioNTech mRNA vaccine was the first to publish positive early results from final stages of clinical trials. An mRNA vaccine has never been approved for use in humans before, and the development took the companies only 10 months.
Another promising mRNA vaccine is that of Moderna, which on 1 December applied to the U.S. Food and Drug Administration (FDA) for emergency use authorisation asking the authority to review an expanded data set, which shows 94.1% efficacy at preventing the disease and 100% efficacy at preventing severe COVID-19 cases. Prior to Moderna, Prizer had made a similar move on 20 November. The FDA will convene its advisory committee on 17 December to review the applications.
Both companies also filed applications for conditional marketing authorisation (CMA) to the European Medicines Agency on the same day, 1 December.
In the meantime, the U.K. government has also formally asked the national regulator to evaluate whether supply of Oxford/AstraZeneca vaccine can be authorised for temporary supply as soon as it receives safety, quality and efficacy data. The UK is the first country in the world to sign an agreement with Oxford University/AstraZeneca for the supply of 100 million doses of the vaccine. It is yet unclear whether the FDA would authorise a COVID-19 vaccine based on overseas data, which is the case of Oxford/AstraZeneca vaccine.
Image credit: claraveritasvia iStock


![Tuberculosis Diagnostics: The Promise of [18F]FDT PET Imaging Tuberculosis Diagnostics: The Promise of [18F]FDT PET Imaging](https://res.cloudinary.com/healthmanagement-org/image/upload/c_thumb,f_auto,fl_lossy,h_184,q_90,w_500/v1721132076/cw/00127782_cw_image_wi_88cc5f34b1423cec414436d2748b40ce.webp)