HealthManagement, Volume 18 - Issue 3, 2018
Solutions for challenging reconstruction of subcutaneous adipose tissue
Induction of blood vessel formation and adipogenic differentiation of fibre-bound human adipose tissue-derived stem cells for soft tissue reconstruction.
The reconstruction of subcutaneous adipose tissue still represents a large challenge in plastic surgery. For a sufficient reconstruction of large-scale damages there is often a lack of autologous tissue. Until now, plastic surgeons were using skin flaps for the reconstruction of soft tissue defects. Depending on the size of the tissue defect, tissue expanders are used to stretch the healthy skin and fatty tissue for several weeks. It also requires few surgical interventions to prepare the wound area and the tissue flaps before transplantation. These processes strongly strain healthy surrounding tissue and are associated with many risks for the patient, eg hypothermia, cardiovascular problems and wound infections. Currently there is no suitable substitution technique that provides a long-term solution in the treatment of soft tissue defects. In small volumes, the autologous transplantation of fatty tissue showed reliable results but the autologous transplantation of larger adipose tissue grafts often results in necrosis and graft resorption. Other approaches in regenerative medicine are marking on artificial fat substitutes. But here again, poor vascularisation and an insufficient supply of nutrients often leads to graft volume reductions up to 60 percent.
Adipose tissue engineering (ATE) instead of autologous tissue substitution, is an upcoming field in regenerative medicine. For these approaches, human adipose tissue-derived stem cells (hASC) are often used. The use of a scaffold in combination with hASC may fix some problems of soft tissue reconstruction in different ways: hASC are known to secrete various growth factors including vascular endothelial growth factor (VEGF) and thus support neovascularisation. Improved blood vessel formation at the site of implantation can improve graft integration and tissue regeneration. On top, these easy accessible adult progenitor cells can easily be isolated from mature adipose tissue and are able to differentiate into the adipocyte cell lineage, what makes them a showpiece for adipose tissue engineering approaches.
For this reason, we thought to engineer adipose tissue grafts using biocompatible, pro-angiogenic scaffold structures in combination with immobilised hASC. Scaffold-induced adipogenic differentiation of immobilised progenitor cells, could in the end help to build up adipose tissue in situ de novo. For this we first selected and engineered suitable fibrous scaffold materials, eg meshes, nonwovens and alginate fibres as well as meshes and gels (Hirsch et al. 2018; Handel et al. 2013; 2012). Suitable scaffolds for adipose tissue regeneration should be biocompatible (according to DIN EN ISO 10993) individually formable, stimulate graft neovascularisation and should preferably directly induce the adipogenic differentiation of incorporated or scaffold bound hASC at the site of implantation.
Our approach for soft tissue regeneration based on human adipose tissue-derived stem cells (hASC) showed distinct angiogenic properties: Standardised in vitro angiogenesis models showed increased angiogenic potential when hASC were seeded onto the implant materials. The quantification of VEGF secretion of scaffold bound hASC followed by HUVEC tube formation assays with conditioned cell culture media showed improved angiogenic properties of the tissue engineered construct. These in vitro results were then correlated and finally confirmed by in ovo CAM angiogenesis assays (Figure 1). In these experiments, the combination of implant materials with undifferentiated hASC showed an enhanced blood vessel formation towards the scaffold material and thus an enhanced angiogenic potential (Handel et al. 2013) .
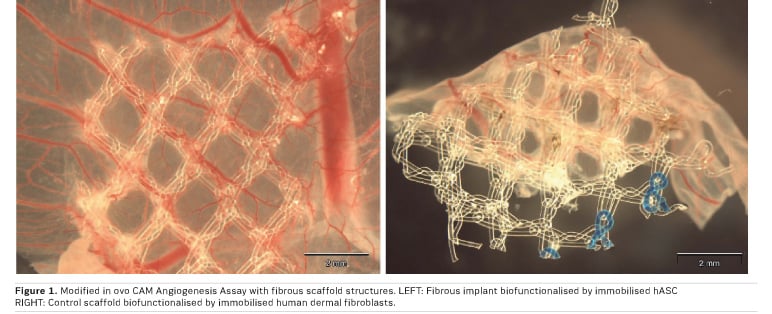
However, with progressing adipogenic differentiation of scaffold bound hASC, proved by microscopy as well as PPARγ gene expression analysis (Handel et al. 2012), gene expression as well as protein secretion of VEGF decreased significantly. To benefit from the pro-angiogenic potential of the progenitor cells, the use of native hASC is advisable.
You might also like: Scientists find key to regenerating blood vessels
Thus, we aimed to engineer biocompatible and pro-angiogenic scaffold materials, which can initiate the differentiation of incorporated or scaffold bound hASC into the adipocyte cell lineage. From a therapeutic point of view, this approach would ensure the required VEGF secretion of hASC and thus the angiogenic potential at the time of implantation followed by the built-up of new soft tissue in vivo. For this reason, we engineered “adipogenic” alginate droplets with incorporated nutrients and differentiation factors, which are intended to induce the adipogenic differentiation of the progenitor cells (Handel et al. 2012). Prior to these experiments, the alginate matrices as well as their production process were verified not to be cytotoxic to hASC (Hirsch et al. 2018). After gelling of the hASC-containing alginate, we obtained spherical alginate microcapsules with an even distribution of cells. With the preparation of an alginate gel by adding nutrients and differentiation-inducing factors directly to the polymer, we obtained scaffolds with an intrinsic adipogenic differentiation capacity. Finally, we could proof this concept by nile red staining of mature adipocytes after cultivation of hASC-containing alginate microcapsules for several days in minimal essential medium (Figure 2).
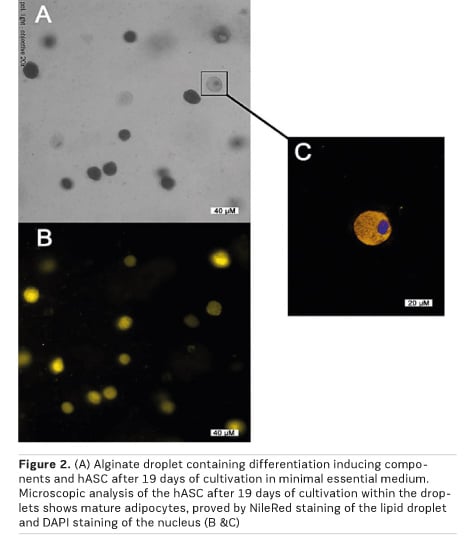
Our results support the theory that scaffold-driven adipogenic differentiation of hASC is feasible for adipose tissue engineering. The procedure shown here advantageously utilises the isolation of hASC from the patient's own adipose tissue and their immediate immobilisation on or into an adipogenic scaffold matrix. In addition to pro-angiogenic properties of the used alginate matrix (Hirsch et al. 2018), we used native hASC for immobilisation, to benefit from their pro-angiogenic potential. Adipogenic differentiation would thus be induced post transplantation within the body of the patient and could be further regulated by the body's own control mechanisms. This approach would significantly shorten the time span from cell isolation to a possible reconstructive surgery and thus simplify the surgical treatment of larger soft tissue defects.
Key Points
- Soft
tissue reconstruction
- Human
adipose-tissue derived stem cells
- Blood
vessel formation
References:
Handel M et al. (2012) Adipogenic differentiation of scaffold-bound human adipose tissue-derived stem cells (hASC) for soft tissue engineering. Biomed Mater, 7: 054107.
Handel M et al. (2013) 45S5-Bioglass®-based 3D-scaffolds seeded with human adipose tissue-derived stem cells induce in vivo vascularization in the cam angiogenesis assay. Tissue Eng Part A, 19: 2703-12.
Hirsch T et al. (2018) Implant for autologous soft tissue reconstruction using an adipose-derived stem cell-colonized alginate scaffold. J Plast Reconstr Aesthet Surg, 71: 101-11.







