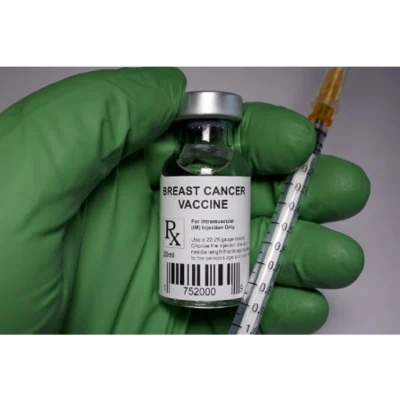
Cleveland Clinic researchers along with Anixa Biosciences have initiated a phase 1 clinical trial to test a vaccine intended to prevent triple-negative breast cancers (TNBC). This trial is a first of its kind.
TNBCs are named so because they test negative for estrogen receptors, progesterone receptors, and excess HER2 protein. Since these are typical targets for breast cancer therapeutics, triple-negative breast cancers carry a poorer prognosis because fewer drugs can target them. TNBCs account for about 10-15% of all breast cancers. As many are basal-type carcinomas with higher grades, TNBCs tend to be fast-spreading. Mastectomy is currently the only prevention measure available in those with high risk.
The vaccine is intended for use in post- or premenopausal women. The vaccine targets α-lactalbumin, a protein involved in milk production. Alpha-lactalbumin is normally expressed during lactation but is not found in ageing tissues post-lactation. Since most TNBCs overexpress the protein, a vaccine-primed immune system can identify and attack emerging TNBC tumours. Given that no autoimmune inflammation has been observed in preclinical trials, it is believed that healthy breast tissue will be spared.
The clinical trial, funded by the U.S. Department of Defense, assesses the maximum tolerated dose and evaluates immune responses to the vaccine. Trial participants will initially consist of 18-24 cancer-free individuals who have been treated for TNBC within the last three years.
The vaccine will be administered as three shots with two weeks between each dose. After determining a safe and effective dose, the trial will expand to include more individuals who are healthy but at high risk.
Anixa Biosciences has an exclusive license to the vaccine technology developed by Cleveland Clinic.
For more Women's Health newsClick here
Sources: Anixa Biosciences, Healio





.jpg)




