HealthManagement, Volume 22 - Issue 3, 2022
Key Points
- The application of AI in medicine has led to greater automation in several fields.
- Radiomics together with AI is useful in diagnostic imaging.
- The transition from qualitative to quantitative imaging could improve the diagnostic and therapeutic approach in several diseases.
Radiomics
Radiomics is a new discipline that allows to extract and analyse a large number of quantitative features taken from medical images acquired with standard techniques (Schapicchio et al. 2021). Once the characteristics of the radiological images are identified and selected, they are stored and then analysed for clinically valid results (Guiot et al. 2022). The term “radiomics” was first introduced in 2012. The birth of this discipline is linked to the need for more personalised medical diagnostics for patients.
Artificial Intelligence (AI), in turn, is a mix of advanced computational algorithms that, once trained, acquire the pattern of data given as input and become able to make predictions about new datasets (Chen et al. 2021; Koçak et al. 2019).
These two disciplines can be used together to manage larger datasets more comprehensively than traditional statistical approaches. The common goal is to extract and analyse as much hidden and meaningful quantitative data as possible to use as a decision support tool (Koçak et al. 2019).
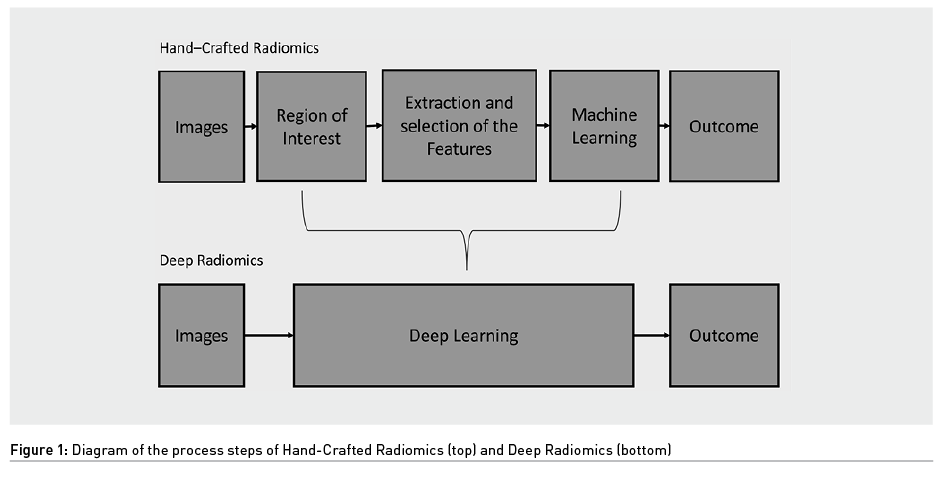
In radiomics there are two different approaches that exploit AI: hand-crafted radiomics and deep radiomics (Figure 1). The first uses Machine Learning (ML), a subcategory of AI, while the second exploits deep learning (DL), a subcategory of ML, from which it takes its name. The difference between these two is that in hand-crafted radiomics human intervention is required, while in deep radiomics the computer provides the result without any human intervention.
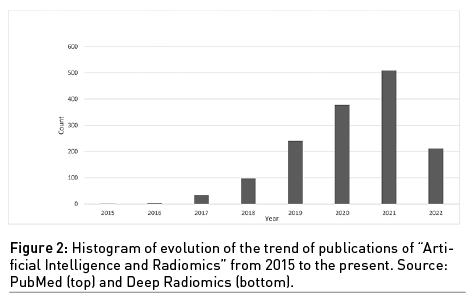
The growing interest in the use of these combined disciplines, AI and radiomics, is evident considering the histogram of the publications over the years obtained by searching “Artificial Intelligence” and “Radiomics” on PubMed (Figure 2).
Ongoing Projects of the UNIFI
A multidisciplinary research group was recently created at the Department of Radiology of the University of Florence (UNIFI). It includes Stefano Colagrande (full Professor and chief), two radiologists, Cosimo Nardi (MD, PhD) and Linda Calistri (MD, PhD), one biomedical engineer, Eleonora Barcali (PhD student) and one rheumatologist, Martina Orlandi (MD, PhD). It also has the support of Professor Leonardo Bocchi, PhD, of the Department of Information Engineering of the UNIFI.
The team thus formed is currently focusing on AI and radiomics in diagnostic imaging. Starting from the literature study on the use of these innovative technologies, the group has conceived four main projects discussed in the following subparagraphs.
A further collaboration, that will not be dealt with in this paper, is being established with Anna Julie Peired (PhD), from the Nephrology unit of the UNIFI, and will focus on developing new methods to assess renal function in paediatric patients using Intravoxel Incoherent Motion (IVIM) parameters from Diffusion Weighted Imaging (DWI) on MR.
COVID-19
The first project discussed is the one from the HORIZON 2020 consortium financed by Innovative Medicines Initiative 2 and titled DRAGON (The RapiD and SecuRe AI enhAnced DiaGnosis, Precision Medicine and Patient EmpOwerment Centered Decision Support System for Coronavirus PaNdemics). This one, whose principal investigator at UNIFI is C. Nardi, concerns the use of AI applied to the COVID-19 emergency. It deals with the development of an ML system to make diagnosis in time and, in the future, be able to better manage upcoming epidemics.
In addition, it aims to predict the evolution of diseases caused by this pathology that are known as long-COVID. The role of the group in this case is focused on the provision of clinical data and CT scans. This project was the first approach of the team to AI applied to diagnostic imaging and the source of inspiration to develop the subsequent projects in other medical fields .
Lung Parenchyma
A second project will investigate the role of MR in the evaluation of the lung parenchyma comparing imaging data provided by CT images with those provided by MR. In particular, it will focus on interstitial lung diseases, especially those secondary to connective tissue disorder. The real challenge is to distinguish mainly inflammatory features from fibrotic damage, in order to select the most appropriate therapy for the patient (immunosuppressant or antifibrotics). In this context, radiomics along with AI could also play a key role in quantifying the percentage of reversible inflamed lesions.
Liver Parenchyma and Function
The third project is currently being evaluated, having been presented to a national programme called PRIN (Projects of Relevant National Interest) 2022, that promotes and funds public research at the national level. The principal investigator is L. Calistri. The project title is “A multifaceted quantitative approach by MRI to stratify liver derangement and dysfunction in chronic liver disease (CLD): a prospective multicentre study by texture analysis, IVIM evaluation and T1 relaxometry” and involves three other Italian universities.
It has three purposes:
- Perform a stratification of different levels of CLD based on the Native T1 value and the T1 Reduction Rate of the hepatic parenchyma after gadoxetic acid disodium (EOB-Gd) administration and the IVIM parameters from DWI on MR.
- Obtain a “quantitative functional liver imaging score” (qFlis) based on the parameters expressed in the previous point to predict CLD outcomes.
- Evaluate CLD grades using liver texture analysis on T1-weighted images to predict patient outcome in comparison to qFlis.
This latter aspect can be achieved by using radiomics to detect changes in individual voxels and their organisation on T1-weighted images to investigate the level of parenchymal imbalance.
In summary, the goal is to calculate a score from the parameters IVIM, T1 Reduction Rate and Texture to semi-quantitatively assess the grade of CLD and predict its outcomes.
Bone Strength
The bone strength, fracture resistance under stress, is strictly related to both mineral density (BMD), and macro/micro geometrical characters. Generally, volumetric BMD can be evaluated quantitively by using Hounsfield Unit calibration of Multi Slice CT (MSCT) images, while for microstructure, the micro-CT is currently the gold standard.
The fourth project of the group aims to evaluate the strength of the bone from cone beam CT (CBCT) images. It relies on the support of EIDO Lab, the joint laboratory founded by the UNIFI together with Imaginalis S.R.L. The purpose is to investigate a new way to make accurate strength assessments from CBCT images using small bones of the extremities, full of trabeculae. The goal is to exploit the AI systems used in radiomics to find a correlation between the trabeculae, their spatial arrangement and their thickness with the strength of the entire structure. The analysis will begin by comparing voxel by voxel the images obtained by MSCT with those obtained by micro-CT and CBCT to find a correlation between them and any change.
Conclusions and Future Developments
The projects described are still in their infancy, but the goal of the group is clear: to contribute to the transition of imaging from qualitative to quantitative and from morphological to functional in the AI era. One of the toughest challenges is to create larger datasets to exploit AI potential at its maximum. The idea of sharing ongoing activities is to create new collaborations and find innovative proposals to form an international multidisciplinary network with a common purpose.
Conflict of Interest
None.
References:
Chen J (2021) A Survey on Applications of Artificial Intelligence in Fighting Against COVID-19. ACM Comput. Surv. 54(8):1-32.
Guiot J et al. (2022) A review in radiomics: Making personalized medicine a reality via routine imaging. Med Res Rev. 42(1):426-440.
Koçak B (2019) Radiomics with artificial intelligence: a practical guide for beginners. Diagn Interv Radiol. 25(6):485-495.
Scapicchio C (2021) A deep look into radiomics. Radiol Med. 126(10):1296-1311.








