HealthManagement, Volume 17 - Issue 2, 2017
HealthManagement.org is pleased to bring you a flavour of Dr. Heywang-Köbrunner’s keynote lecture to the EUSOBI 2016 annual scientific meeting, to celebrate the 30th anniversary of contrast-enhanced breast MRI.
In 1984, with colleagues I first began to investigate magnetic resonance imaging (MRI) of the breast without contrast to discriminate between benign and malignant lesions using different pulse sequences, calculated T1 values, T2 values and proton density values (Hey wang et al. 1987). We found that in some lesions (which consisted of different tissue components) a characteristic internal structure was visible on MR imaging, reflecting their histopathologic structure. For most lesions with irregular contours a discrimination based on signal intensities or calculated T1- and T2-values did not seem possible, however.
Based on this experience I suggested studying the potential of MRI using contrast agents. The radiologists I spoke to, including the head of our department, were sceptical, and t hought t hat M R contrast was too expensive and too invasive for breast examinations. After many discussions the first supporters who recognised the potential of this idea were researchers and managers in the industry. These people, including Dr. H.P. Niendorf, Prof. Dr. U. Speck, Dr. W. Clauss and Dr. Hans-Joachim Weinmann from the German pharmaceutical company Schering AG, carried out safety studies on gadolinium-DTPA (eg Niendorf et al. 1991). Only based on their support did our very first studies become possible.
Contrast-Enhanced MRI: The Pioneer Patients
The very first study on contrast-enhanced MRI (CE MRI) only included 10 patients, who were examined in 1985. One of these patients was a 25-year-old mother with a 3cm breast cancer on the right side. The left breast appeared normal with palpation, ultrasound and mammography. The MRI showed an ipsilateral 3 cm fast-growing cancer, and by chance a second contralateral small scirrhous cancer was detected. Even though this young mother was very brave and confident in the potential of medicine, the patient did not survive. But because of her findings continuation of our research became possible and further examinations and studies followed. Today probably many other women are alive because of her.
First Steps…
During the first studies already we noted the value of CE MRI for distinguishing dense fibrous tissue and scar tissue from carcinoma. The first publications on CE MRI also included a first case of an enhancing ductal carcinoma in situ (DCIS) (Heywang et al. 1986). The fact that DCIS enhanced with the contrast agent indicating an increased perfusion was astonishing, since DCIS is just a precursor of breast cancer, which in up to fifty percent of cases develops to become breast cancer, and it was not expected to be well perfused. Formerly it was expected that only invasive breast cancers could be associated with increased vascularity and perfusion..
In 1987/88 new pulse sequences became available, which allowed measurement of the uptake of the contrast agent Gd-DTPA at several time points after injection. This allowed evaluation of the speed of uptake, to observe the wash-in and wash-out of contrast agent with time and thus measurement of so-called dynamic enhancement curves for different tissues (Heywang et al. 1988). We found that in some cases a better differentiation seemed possible early after contrast medium than on the later scans. At that time Dr. W.A. Kaiser also began investigations into this method (Kaiser and Zeitler 1989).
Both groups of researchers showed that a variety of
enhancement curves could be observed in different tissues. Some benign and
malignant lesions could often (but not always!) be better distinguished by including
this added information.
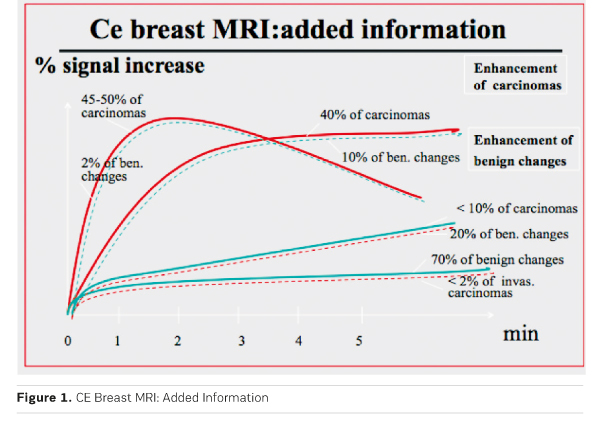
Overall contrast enhancement (uptake) gives functional information about the pathophysiology of the underlying tissue. Increased and early uptake, as observed in about 90% of invasive breast cancers and in up to 50% of DCIS is caused by increased vascularity, vascular permeability and increased interstitial space. Studies have shown that growth of invasive tumours beyond a size of 2-3 mm requires such increased and changed vascularity. Growth of such tumour vessels is caused by so-called growth factors, which are produced by the tumours. Approximately half of the malignant tumours also demonstrate an early washout. If present, this may be an important indicator of malignancy. Until today the above-mentioned observations constitute the basic information, which can be gained from contrastenhanced breast MRI .
See Also: Breast MRI: The New Standard in Breast Imaging?
Research Continued
The late 1980s and onwards saw more publications, looking both at the benefits and the pitfalls (Heywang et al. 1988; Heywang-Köbrunner 1990; Heywang- Köbrunner and Beck 1996; Kaiser 1993; 2008).
When comparing the information that can be obtained by mammography and ultrasound we found that MR imaging provides different complementary information and thus proved “beneficial as a supplement in selected, diagnostically difficult cases”. These results were published in a study of 150 patients with 167 biopsy-proved lesions examined by MRI with and without contrast and by the other imaging modalities (Heywang et al. 1989).
In 1996 we highlighted that certain pulse sequences (so-called opposed-phase sequences) cannot be used for contrast-enhanced MRI of the breast. The reason is that the signals of surrounding fat and enhancing glandular tissue may cancel each other with this technique. This observation, which predominantly concerns small lesions such as DCIS or small invasive cancers, was quite important, since otherwise these early malignancies might go undetected (Heywang-Köbrunner et al. 1996).
One major topic of our studies concerned investigation of the value of contrast-enhanced MRI for various diagnostic questions and indications:
In a 1990 study we looked at 60 patients with postoperative scarring, with (30) and without (30) silicone implants, including 28 patients with obvious normal or abnormal findings and 32 diagnostically difficult patients, who were referred to MR because of uncertain mammographic and/or clinical findings (Heywang et al. 1990). In the diagnostically difficult cases, CE-MRI proved helpful in 23/32 cases. While scarring early after surgery often enhanced and thus showed confusing results, scarring older than 6 months usually did not enhance. As most invasive carcinomas larger than the slice thickness enhance significantly, CE MR allowed excellent discrimination between scarring older than 6 months and malignancy.
We further investigated the technique for women with breast silicone implants, and found that 4/13 recurrences were detected by MRI only. In addition, MRI correctly diagnosed scar tissue in all cases with indeterminate findings. We therefore recommended contrast-enhanced MRI in patients with diagnostic problems or high risk of recurrence after silicone implants (Heinig et al. 1997). This has today become an indication for MRI, which is accepted by insurance organisations in most industrialised countries.
In a 1993 study of patients after tumourectomy and radiation therapy we investigated the enhancement of tissue during variable time intervals after therapy with CE MRI (Heywang-Köbrunner et al 1993). Up to nine months after therapy, differentiation between posttherapeutic changes and recurrence was often difficult, because both the tumour and scar tissue enhanced with contrast agent. We therefore recommended that CE MRI not be used before nine months after radiation therapy; however, after 18 months it regularly proved to be a valuable additional tool. This was confirmed by another follow-up study of our group in 1998 (Viehweg et al.). In this larger study of 207 patients who had undergone lumpectomy we concluded that in the first year after therapy, CE MRI is only indicated in selected cases. Later than 12 months after radiation therapy CE MRI contributed significant additional information. It allowed much better distinction of post-therapeutic fibrosis from recurrent cancer, and detected recurrent disease with more sensitivity and at an earlier stage. This, too, has now become a widely accepted indication for breast MRI.
In 2002 we published a study that showed that short-term anti-oestrogen medication could suppress part of the unspecific enhancement seen on breast (Heinig et al. 2002). We had hoped that this information might be used diagnostically in the future. However, until today anti-oestrogen medication is only applied therapeutically.
Parallel to the above studies reported by our group other research groups started to investigate CE MRI of the breast and contributed various publications. Most early publications stem from 1993 and 2003. In 1996- 1999 the first international multicentre study took place in 11 sites in the Netherlands, Belgium, Germany, the USA, Sweden and France. It was published in 2001 (Heywang-Köbrunner 2001). This showed that it is possible to use a widely available standardised MR technique and define statistically founded interpretation rules. The sensitivity achieved with a low specificity ranged around 97% or around 91% with high specificity level.
The above multicentre study was soon followed by a second multicentre study conducted between 1998 and 2001 in Germany and the USA , published in JAMA in 2004. It analysed MRI use as a potential replacement for biopsy (Bluemke et al. 2004). The researchers concluded that breast MRI has high sensitivity but only moderate specificity independent of breast density, tumour type, and menopausal status. However, they did not recommend using MRI alone instead of tissue sampling, a result which is widely accepted, even today. Even though MRI is the most sensitive method for detecting invasive breast cancer, no imaging method is perfect. Thus in cases with suspicious findings, percutaneous breast biopsy, which meanwhile has been developed as a minimal invasive, very reliable and widely accepted method, is the gold standard for further assessment of imaging-detected lesions.
Breast MRI Takes Off
Following the two multicentre studies described above, the “take-off” of breast MRI can be dated to the new millenium. Before 2000 there are fewer than 500 hits for MRI of the breast in PubMed. After that date there are more than 10,000 hits.
Peters and colleagues conducted a m eta-analysis of 44
studies of MRI to diagnose breast lesions (Peters et al. 2008) and found
overall sensitivity of 90% and specificity of 72%. Systematic reviews of randomised
controlled trials of preoperative MRI found approximately 20% additional
detection of lesions in some studies (Plana 2012; Turnbull 2010; Peters 2011; Houssami
2014; Fancellu 2015; DiLeo 2015). However, the studies also found that MRI is
also associated with a high number of false positive calls and with possible over-detection
and potential overtreatment.
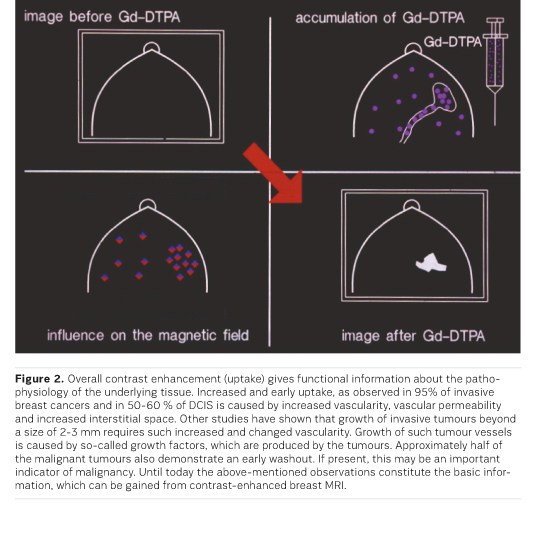
Based on the initial work described above and the existing possibility for further assessment of MR-detected lesions, MRI has been used and investigated for an increasing number of indications. CE MRI has proven most valuable for part of the indications; many indications, however, can still be solved without MRI. Indications for which MRI is not recommended include screening of women at low risk (which can mostly be solved by mammography) or work-up of suspicious lesions, for which minimal invasive biopsy methods are considered methods of choice.
Indications for Contrast-Enhanced MRI
So, after 30 years of contrast-enhanced MRI, how should this technique be used today?
MRI Screening of High-Risk Women
After MRI became more and more accepted as a very sensitive method for women with a high risk of breast cancer, CE MRI has become the most important imaging modality. The work is based on important research from several international groups. The most important early publications and their results are shown in Table 1.
In many women who are at high risk breast cancer grows earlier than in the usual population. During younger age the breast tissue is denser and mammography is less sensitive. Also, certain tumour types occur in these women, which are not reliably detected by mammography or ultrasound. This explains why MRI is superior to ultrasound and mammography in sensitivity in these women (Table 1).
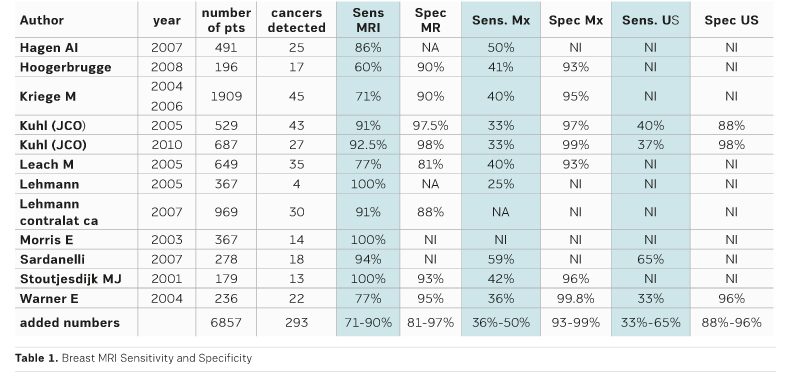
Two systematic reviews confirm the high sensitivity of MRI performed with mammography in these women (Lord et al. 2007; Warner et al. 2008).
A third meta-analysis h as meanwhile been published by Phi and colleagues. They assessed MRI screening in BRCA mutation carriers over 50 years of age using individual patient data meta-analysis, and found that in women over 50 years sensitivity was significantly increased, as with the under 50 years age group (Phi et al. 2015).
To date evidence is clear that MRI is by far the most sensitive method in these women. And this is accepted, even though specificity of MRI is lower than that of mammography and even though long-term data concerning survival or mortality reduction are not (yet) available.
The evidence for long-term outcomes is still being collected. One study that looked at 10-year survival of 496 women at high risk of breast cancer, who were screened with MRI , found that the cancers identified were at an early stage, and the annual breast cancer mortality rate in the study was very low (Passaperuma et al. 2012). Evans et al. (2014) found no difference in 10-year survival between women screened with MRI and mammography and mammography-only groups. However, survival was significantly higher in the MRI - screened group (95.3%) compared to no intensive screening.
Based on present knowledge, MRI screening, mostly combined with mammography, is recommended to those women at high risk, who do not opt for surgical removal of the breast tissue. Fortunately, familial high risk only makes up about ten percent of all breast cancers, and most breast cancers to date can be detected early using mammography screening, sometimes supplemented by other methods.
Women at Intermediate Risk of Breast Cancer
The group of
women at intermediate risk is much larger than the groups of women at high
risk. It includes women with some increased risk of breast cancer in the
contralateral breast, as a small proportion of women diagnosed with unilateral
breast cancer go on to develop cancer in the other breast. Researchers have
shown that MRI can detect contralateral breast cancer soon after diagnosis in
the other breast when it was missed by mammography and clinical examination, but
the technique may also lead to a high number of false positive calls (Lehman et
al. 2007; Brennan et al. 2009). A single-centre study by Kim and colleagues
(2013) showed a single MR imaging screening examination of the contralateral
breast in women with unilateral breast cancer increased synchronous cancer
detection and was associated with decreased diagnosis of contralateral cancer
within 45 months, the “grey zone”.
Also in this “grey zone”, where the evidence is not yet clear, are MRI screening for women with a history of lobular carcinoma in situ (LCIS ) or atypical ductal hyperplasia (ADH ): the IAR C working group that met in 2014 found “limited evidence” (IAR C 2016). For women at intermediate family risk of breast cancer insufficient data exist on the usefulness of MR screening for breast cancer.
MRI Imaging and Neoadjuvant Chemotherapy
Several studies
have evaluated MRI imaging for assessing residual tumour following neoadjuvant chemotherapy,
and its concordance with pathology (Marinovich et al. 2013a). In preoperative
assessment of women who receive neoadjuvant chemotherapy (NA C) MRI has been
shown to be more effective than mammography in assessing residual tumours
(Marinovich et al. 2013b). When evaluating early response to neoadjuvant
chemotherapy (NA C) PET has been shown to be slightly more effective than MRI ,
but MRI had better results for imaging after completion of NA C (Sheikbahael et
al. 2016). The addition of diffusionweighted imaging (DWI) to CE-MRI gives
comparable results (Wu et al. 2013)
Detection of Recurrent Breast Cancer
MRI to assess recurrence after breast-conserving therapy is more useful according to one meta-analysis comparing PET with other imaging modalities (Pan et al. 2010). MRI is most accurate in detecting breast cancer recurrence and contralateral breast cancer, although the evidence is limited (Robertson et al. 2011).
MRI for Diagnosis and Problem Solving
The aim of all breast screening is to minimise false positives. MRI has been evaluated for its role in assessing suspicious breast lesions. Medeiros et al.’s meta-analysis of literature up to 2010 showed MR as a useful preoperative test to predict the diagnosis of breast lesions (2011). Bennani-Baiti et al. (2016), in their meta-analysis, found excellent results from MRI in diagnosing non-calcified uncertain breast findings found via conventional imaging.
MRI Tomorrow?
Several recent and ongoing trials address the addition of MRI in screening women with a familial risk of breast cancer and dense breasts (Saadatmand et al. 2012; DENSE trial - clinicaltrials.gov/ct2/show/ NCT01315015; Emaus et al. 2015). So investigation of MRI for risk-adapted screening will become an important future topic. Also, the use of MRI to monitor less aggressive treatment options might gain importance.
Conclusion
In the future, we need to optimise existing technologies and test new technological possibilities, including DWI, diffusion tensor imaging and diffusion weighted whole body imaging (DWIBS ) and more to come... Will CE MRI endure? It has contributed significant progress to breast imaging. However, there is potential for further improvement. We always need to weigh the advantages and disadvantages. Whatever we use, the ultimate goal is the care of our patients.

Prof. Dr. Sylvia Heywang-Köbrunner is recognised internationally for her expertise and innovation in the field of breast diagnostics and mammographic screening. She pioneered contrast-enhanced MRI for breast imaging, and has registered several patents for biopsy techniques. Dr. Heywang-Köbrunner directs the Munich Breast Diagnostic Clinic and runs the Mammography Reference Centre in Munich, one of the five centres that, together with the Mammography Collaboration Community, are responsible for the introduction, quality assurance, advanced training and accompanying research in German mammographic screening. In this capacity, she also manages the Screening Unit Munich South. Dr. Heywang-Köbrunner was awarded the Gold Medal of the European Society of Breast Imaging at its annual scientific meeting in Paris in October 2016 in recognition of her scientific achievements in the field of breast imaging.
Appendix
The following images are reproduced by permission from: Heywang-Köbrunner SH, Dershaw DD, Schreer I (2014) Diagnostic breast imaging: mammography, sonography, magnetic resonance imaging, and interventional procedures. 3rd ed. Stuttgart: Thieme. DOI: 10.1055/b-002-9286. Print ISBN 9783131028939. Online ISBN 97831504111
They illustrate the excellent response of a grade III triple negative invasive ductal carcinoma to neoadjuvant chemotherapy.
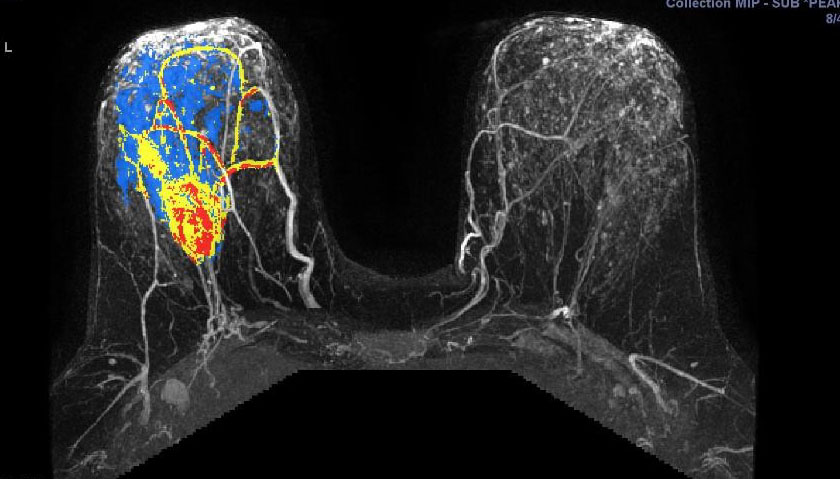
Figure 5.17a - a) shows activity of a fast growing
(G3) tumour before chemo. This pretreatment scan is a 3D maximum intensity projection (MIP) postcontract image. (In the axillary tail, part of a normal lymph node is seen.)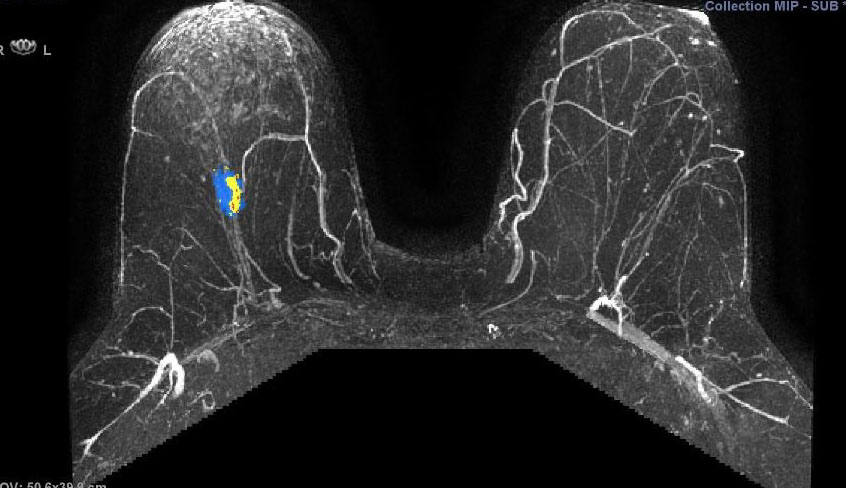
Figure 5.17 b: After chemo, there is no more activity in the
tumour. This follow-up scan during treatment is a 3D MIP postcontrast image. After two cycles of chemotherapy, the mass is already markedly reuced in size and the enhancement pattern is predominantly slow rising.
(CAD-generated colour map: red is rapid strong enhancement, and washout; blue is moderate enhancement, and plateau; yellow indicates slow rising enhancement.)



