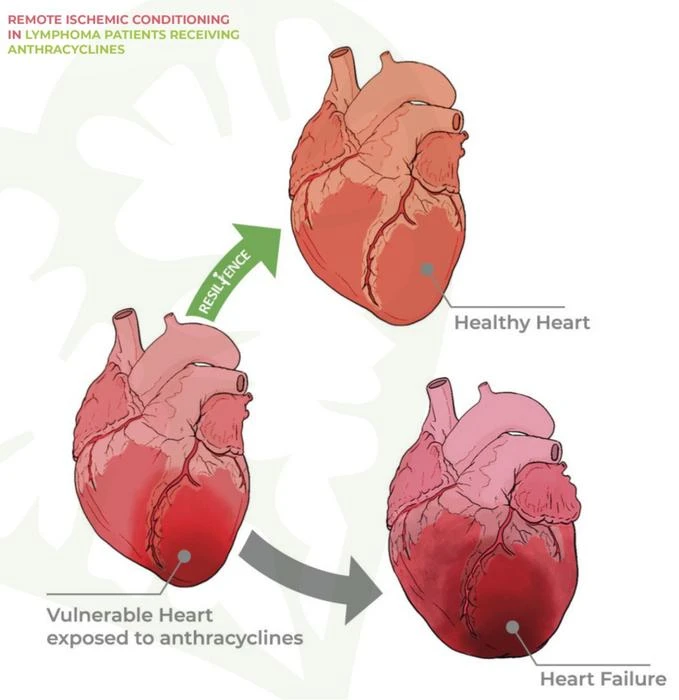
The RESILIENCE clinical trial aims to assess the safety and effectiveness of remote ischaemic conditioning (RIC) in preventing the cardiotoxic effects of anthracycline-based chemotherapy in lymphoma patients. This trial is expected to provide critical insights and potential solutions for a major clinical challenge.
Anthracycline-induced cardiotoxicity remains a significant concern for cancer patients, with few effective protective treatments available. Preclinical studies have shown promising cardioprotective effects of RIC, which involves briefly interrupting blood flow to a limb, typically an arm, to protect vital organs such as the heart from damage caused by chemotherapy. The RIC process uses an arm cuff, similar to a blood pressure cuff, which inflates for 5 minutes and then deflates, repeating up to four times in a session.
Funded by the European Commission’s H2020 Programme, the trial aims to reduce the incidence of heart failure in cancer survivors, improving their quality of life. It is coordinated by the Centro Nacional de Investigaciones Cardiovasculares (CNIC), led by Dr. Borja Ibáñez, and involves key partners like the European Society of Cardiology (ESC), which plays a pivotal role in project coordination and stakeholder engagement. Other notable partners include research institutions and hospitals across Spain, Portugal, France, Germany, the Netherlands, and Denmark, with Philips Healthcare providing the imaging technology. The trial also has the support of the Lymphoma Coalition Europe patients’ association.
Cardiovascular complications are a risk for cancer patients, particularly those treated with anthracyclines, which are effective against several cancers, including lymphoma, breast cancer, and leukaemia. However, these drugs can cause cardiotoxicity, leading to chronic heart failure. In Europe, more than 3 million cancer patients are treated with anthracyclines annually, with over one-third developing treatment-induced cardiomyopathy.
The RESILIENCE trial seeks to test RIC’s cardioprotective potential in a clinical trial involving 608 lymphoma patients at high risk of cardiotoxicity. So far, over 220 participants have been enrolled. The trial also aims to evaluate the effectiveness of advanced cardiac magnetic resonance (CMR) imaging techniques in detecting early signs of cardiotoxicity.
Participants will undergo RIC or a placebo procedure weekly during chemotherapy. They will have CMR scans at the beginning of the study, after three chemotherapy cycles, and two months after completing treatment. These scans will monitor changes in heart function, particularly left ventricular ejection fraction (LVEF), a key measure of cardiac health. Secondary outcomes will include cardiotoxicity-related events, such as significant drops in LVEF.
The trial will compare the predictive capabilities of advanced CMR technology with established markers like left ventricular deformation and cardiac injury biomarkers. The trial also aims to validate a new magnetic resonance sequence for ultra-rapid cine acquisition, which could reduce scan times for this vulnerable population.
The RESILIENCE trial represents a crucial step forward in developing cardioprotective strategies for cancer patients at high risk of anthracycline-induced cardiomyopathy. All trial participants have characteristics that put them at increased risk for this serious cardiac complication. The trial is funded by the European Commission.
Source: CNIC
Image Credit: CNIC