HealthManagement, Volume 2 - Issue 2, Winter 2008
Improving the Management of Thromboembolitic Prevention in Routine Practice
Anticoagulants are recommended for a wide variety of indications, including the prevention of venous thromboembolism (VTE; comprising deep vein thrombosis [DVT] and pulmonary embolism [PE]), the treatment of VTE, stroke prevention in patients with atrial fibrillation (AF) and secondary prevention of major ischaemic outcomes in patients with acute coronary syndrome (ACS). This article sums up the recent developments in anticoagulants and discusses the implications for the management of cardiovascular conditions.
Established
anticoagulants include unfractionated and low molecular weight heparins (UFHs
and LMWHs), vitamin K antagonists (VKAs) and, more recently, the synthetic
pentasaccharide fondaparinux (see table 1). Until recently VKAs, including
warfarin, were the only approved oral anticoagulants but they have a narrow
therapeutic window, require routine laboratory monitoring and have multiple
food and drug interactions. The development of novel anticoagulants has focussed on small moleculesthat target only one coagulation factorspecific for the final common pathway inthe coagulation cascade.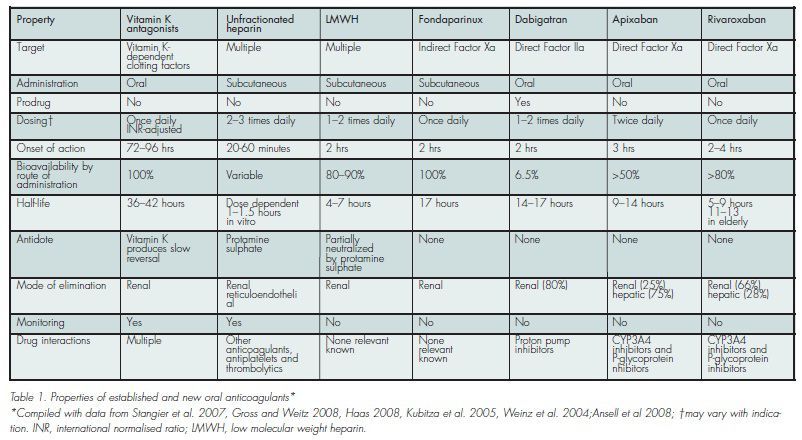
New Anticoagulants
At present, there is one orally administered direct thrombin
inhibitor (DTI) available on the market, dabigatran, launched in Europe and
Canada for the prevention of VTE in adult patients who have undergone elective
total hip and knee replacement (THR and TKR) surgery. It is under investigation
for VTE treatment, and prevention of cardioembolic events in patients with
chronic AF. Other agents, such as AZD0837 and direct FXA inhibitors are also in
development. Proof of concept for targeted FXA inhibition, was provided by
fondaparinux, although this agent requires parenteral administration.
Dabigatran
Pharmacology
Dabigatran etexilate is an orally available prodrug that is converted rapidly to its active form dabigatran. It is a potent, competitive and reversible DTI.
Clinical Trials
In the RE-NOVATE and RE-MODEL studies, dabigatran 150 mg and 220 mg were both non-inferior to enoxaparin 40 mg once daily for the prevention of VTE in adults undergoing elective THR and TKR, respectively. Dabigatran had a similar safety profile to enoxaparin in both trials. The RE-MOBILIZE trial compared dabigatran with the North American enoxaparin regimen (30 mg twice daily) for the prevention of VTE after TKR. Dabigatran failed to demonstrate non-inferiority to the twice-daily enoxaparin regimen.
The
clinical trial programme also includes studies of dabigatran in treatment of
acute DVT and PE (RECOVER), prolonged prevention of recurrent VTE (RE-MEDY and
RESONATE) and the prevention of stroke and systemic embolism in patients with AF
(RE-LY). The RE-DEEM study will evaluate safety and indicators of efficacy with
four doses of orally administered dabigatran etexilate in ACS patients who have
had a myocardial infarction, and are therefore at high risk for further
cardiovascular events.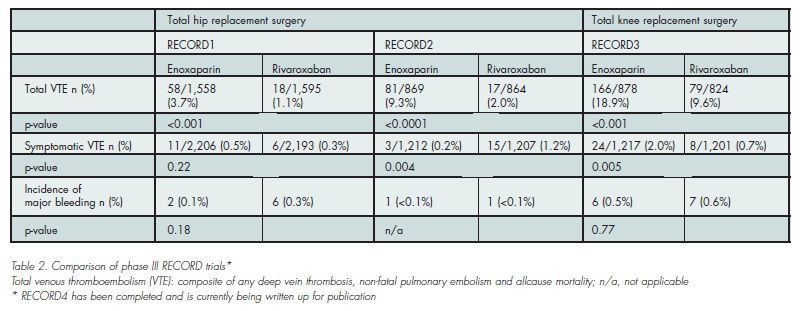
Apixaban
Pharmacology
Apixaban is an oral, highly selective, direct inhibitor of free and prothrombinase- bound FXa.
Clinical Trials
Three large phase III studies are evaluating the safety and
efficacy of oral apixaban in patients undergoing elective TKR (ADVANCE-1 and
-2) and THR (ADVANCE-3). Results of ADVANCE-1 indicated that the rate of the primary endpoint of this study
was numerically similar to that observed with enoxaparin (9.0% vs 8.9%,
p=0.064), but did not meet the pre-specified statistical criteria for
non-inferiority compared with the US dosing regimen of enoxaparin.
The apixaban clinical trial programme is also investigating
apixaban for VTE prevention in medically ill patients (ADOPT), VTE treatment
(AMPLIFY and AMPLIFY-EXT) and stroke prevention in AF (ARISTOTLE and AVERROES).
A phase II, randomised, doubleblind, placebo-controlled, multi-centre clinical
trial (APPRAISE-1) to assess the safety of apixaban in patients (n = 1,715) with
recent ACS is ongoing.
Rivaroxaban
Pharmacology
Rivaroxaban is an oral, direct FXA inhibitor that effectively inhibits free FXA activity, prothrombinase-bound and clot associated FXA activity. Rivaroxaban has predictable dose-dependent pharmacokinetics and pharmacodynamics.
Clinical Trials
An extensive phase II programme indicated that rivaroxaban can be given to patients without dose modification for age, weight, gender and mild to moderate renal impairment. Results from these studies showed that total VTE (the composite of any DVT, non-fatal PE and all cause mortality) occurred in significantly fewer patients receiving the rivaroxaban regimens compared with those receiving the enoxaparin regimens with similar rates of major bleeding.
When compared to the US regimen of enoxaparin (30 mg bid starting 12 - 24 hours after wound closure) rivaroxaban had superior efficacy for the prevention of VTE after TKR, without significantly increasing the risk of bleeding. The phase II and phase III programmes also include studies evaluating rivaroxaban for VTE treatment (EINSTEIN DVT and EINSTEIN PE), longterm prevention of recurrent symptomatic VTE (EINSTEIN EXT), VTE prevention in medically ill patients (MAGELLAN), and the prevention of stroke in patients with AF (ROCKET AF).
Finally, the ATLAS ACS TIMI 46 phase II dose-finding study is evaluating rivaroxaban in patients with ACS treated with acetylsalicylic acid (ASA) alone or ASA + thienopyridine.
Rivaroxaban has recently received approval in Canada and the EU
for the prevention of VTE in patients undergoing elective THR or TKR surgery.
Implications for Management of Cardiovascular Conditions
These new anticoagulants may change the therapeutic approach to treatment and prevention of VTE. LMWHs re-quire parenteral administration and are usually bridged to oral warfarin for long-term outpatient therapy. With oral anticoagulants, VTE prophylaxis after surgery could be initiated orally and patients could be discharged on the same oral medication, avoiding the need for self-injection or nurse visits for drug administration.
AF is a major risk factor for stroke. Currently, ASA and VKAs are the only available antithrombotics for stroke prevention in AF. Long-term stroke prevention with the new oral anticoagulants could potentially be more convenient for the patient. Furthermore, an oral anticoagulant that can be taken in a fixed, oncedaily, dose with no need for monitoring might offer significant potential in the management of stroke prevention in AF.
Cost analyses suggest that anticoagulation is most effective and results in the greatest overall cost savings when applied to populations at highest risk for thrombotic events.
Preliminary analyses suggest that significant savings might be realised from oral administration compared with drugs administered parenterally that also require self-injection or nurse visits following discharge. Anticoagulants that offer greater efficacy or safety than current standards of care might achieve further cost savings due to a reduced incidence of thromboembolic events or haemorrhages, and corresponding reductions in recurrence and long term complications.