An introduction to the European study on clinical DRLs for x-ray medical imaging (EUCLID).
The EUCLID project was launched by the European Commission to collect data needed for the establishment of DRLs for the most important x-ray imaging tasks, from the perspective of radiation protection, and to specify up-to-date DRLs for these clinical tasks.
The European Commission (EC) launched the European study on clinical diagnostic reference levels (DRLs) for x-ray medical imaging (acronym: EUCLID) project on 1 August 2017 to provide up-to-date clinical DRLs. The main objectives of the project are to:
a) conduct a European survey to collect data needed for the establishment of DRLs for the most important x-ray imaging tasks in Europe from the perspective of radiation protection; and,
b) specify up-to-date DRLs for these clinical tasks. Moreover, a workshop will be organised to disseminate and discuss the results of this project and to identify need for further action. Detailed information about the project is available on the EuroSafe Imaging website (eurosafeimaging.org/euclid)
DRLs should be specified for clinical indications, because different image quality is needed for different clinical indications of the same anatomical area. Kidney stone evaluation, for example, can be performed by using lower radiation doses than those used in evaluation of appendicitis, because detection of high-contrast structures is affected less by high image noise than low-contrast structures.
To fulfil its objectives, EUCLID relies mainly on:
- An External Advisory Panel (EAP) that has been set up to be consulted on the main project activities and outcomes
- A Scientific Board (SB) that has been set up to verify the data sources used; and,
- Α network of EuroSafe Imaging (http://www.eurosafeimaging.org/) hospitals and their experts.
The project is divided into five work packages (WPs). Each WP covers specific tasks leading to the common objective of carrying out a European study on clinical DRLs for x-ray medical imaging. The five WPs are:
- WP1 is responsible for the management and general coordination of the project, as well as for dissemination of project activities and outcomes.
- WP2 is responsible for the identification of the procedures and clinical indications for which DLRs will be established, as well as for the review of existing DRLs.
- WP3 is responsible for conducting a European DRL survey for computed tomography (CT) and interventional radiology, following a predefined methodology.
- WP4 is responsible for specifying/determining up-to-date European clinical DRLs for Europe for the protocols/imaging tasks identified under WP2 and stakeholder consultation/validation of the DRLs.
- WP5 will consist of organising a workshop to disseminate and discuss the results of the project with Member States and relevant national, European and international stakeholders. The workshop should help in identifying any need for further national and local action on establishing, updating and using DRLs.
EUCLID WP2 has contacted national competent authorities to request information about existing clinical DRLs and has performed an extensive literature review to identify studies on clinical DRLs. Results show that only five countries have some clinical indication-based DRLs for CT and only three countries intend to develop clinical indication-based DRLs in the near future.
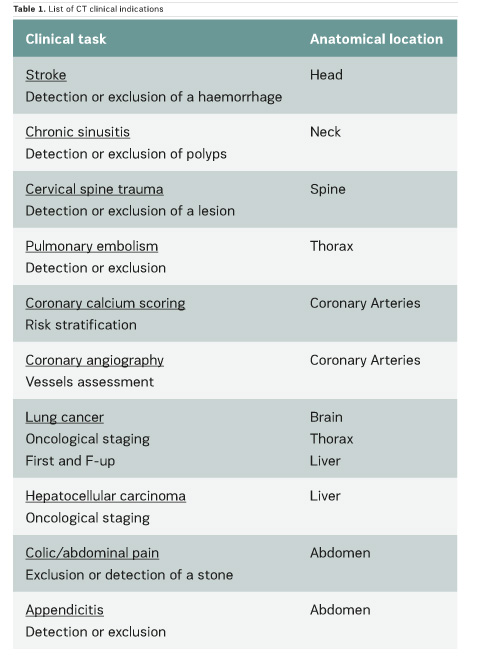
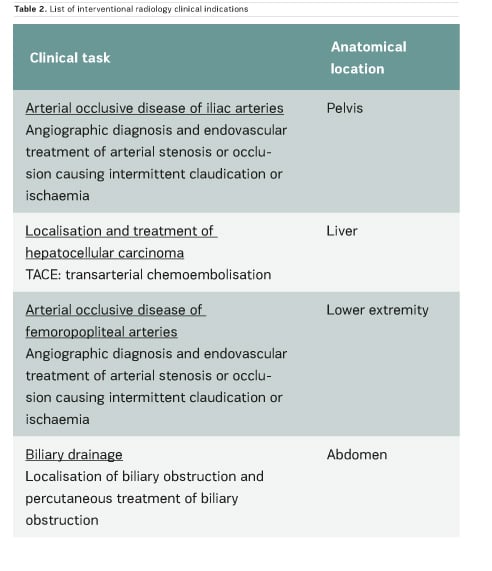
In the scientific literature, few articles provide clinical CT DRLs, and usually for a single indication only. Regarding fluoroscopically-guided procedures, the number of existing DRLs is very limited: for example, only one country has established DRLs for hepatic embolisation and there are no DRLs for endovascular aneurysm repair (EVAR) procedures. Furthermore, few important multicentre studies on CT and interventional DRLs have been published very recently (Ruiz-Cruces et al. 2016; Etard et al. 2017; Tuthill et al. 2017; Kanal et al. 2017). Studies on interventional DRLs (Ruiz-Cruces et al. 2016; Etard et al. 2017) support the value of evaluating the level of complexity of interventional radiology procedures. The EUCLID findings show that clinical DRLs are needed for many CT clinical indications and fluoroscopically-guided procedures. Tables 1 and 2 show CT clinical indications and interventional radiology procedures for which DRLs will be established by the EUCLID project.
WP3 has developed a survey to collect data required for the establishment of DRLs from hospitals across Europe. The basic structure of the survey questionnaires is shown below:
Computed Tomography (CT):
- General instructions
- Contact information
- Scanner specifications and protocol
- Equipment data
- Quality control data
- Protocol data
- Patient data
- Dose data
- Image quality data
Interventional Radiology (IR):
- General instructions
- Contact information
- X-ray system specifications and protocol
- Equipment data
- Quality control data
- Protocol data
- Patient data
- Dose data
- Image quality data
- Data for the grading of procedure complexity
- Data for the assessment of operator skills
The final version of the questionnaires was produced taking into consideration feedback from the SB and the EAP members, recent national and international surveys on DRLs, recent publications on interventional radiology DRLs, and the necessity to assess the complexity of the fluoroscopically-guided procedures.
The European Institute for Biomedical Imaging Research (EIBIR) has established a professional platform for data collection: the EIBIR Electronic Data Capture Platform (eibir-edc.org). This platform uses the REDCap software package to facilitate the collection and management of study data. It can collect almost any type of research data, from numerical values or text to DICOM images, and all data is transmitted through a secure connection. In particular, the export function of REDCap fits well with EUCLID WP4 needs, since responses can be exported to Excel or professional software packages for data analysis. Centres participating in EUCLID have received instructions on the use of the EIBIR platform and started uploading data for DRLs determination in June 2018.
Conclusion
Different image quality is needed for different clinical indications of the same anatomical area and, therefore, DRLs should be specified for clinical indications. The European Commission (EC) launched the EUCLID project to provide up-to-date clinical DRLs. As a first step, EUCLID has developed a survey to collect data required for the establishment of DRLs from hospitals across Europe.

Key Points
- The European Commission launched the EUCLID project in August 2017 to provide up-to-date clinical DRLs for x-ray medical imaging
- Lists of CT clinical indications and interventional radiology procedures for which DRLs will be established have been created
- EUCLID has also developed a survey to collect data required for the establishment of DRLs from hospitals across Europe.
- A workshop will disseminate and discuss the results of the project results and identify the need for further national and local action on establishing, updating and using DRLs