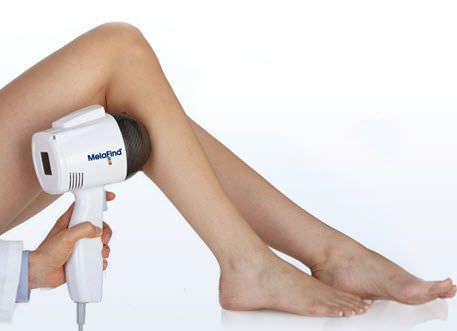
The FDA has agreed to the MELA Sciences’ MelaFind® Pre-Market approval application to be used in the United States. The MelaFind® (medical technology) can now be sold off in the European Union as MELA Sciences has been permitted with the CE Mark approval after its completion of a successful conformity assessment procedure. MelaFind® can be used in the U.S under certain circumstances set by the FDA and MELA Sciences. This worldwide popular technology can be used on clinically atypical wounds caused by cutaneous pigmentation with one or more clinical or historical features of melanoma but it cannot include those with a clinical treatment of melanoma or likely melanoma. When biopsy needs to be done, the dermatologists require more precise information to go ahead with biopsy which can be easily performed by MelaFind® technology. MelaFind® technology should be avoided at any cost in order to confirm a clinical diagnosis of melanoma. MelaFind® should be used by professional physicians or dermatologists only who are trained in the appropriate use of MelaFind® technology and the clinical diagnosis and management of skin cancer.
a:1:{i:0;a:2:{s:4:"name";s:23:"Type of skin diagnosis:";s:3:"val";s:21:"skin pigment analysis";}}