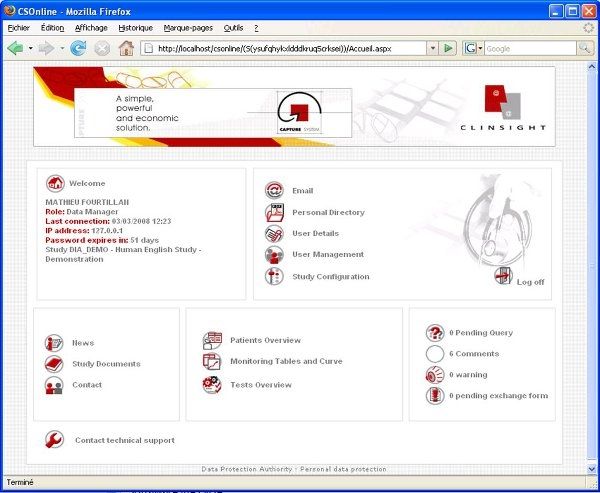
User-friendly and visual solution: optimizing the day-to-day work of the data managers, Investigators, CRAs, Statisticians and Project Managers. Flexible solution: combining direct online data entry (eCRF) and standard data entry (paper CRF). - Management of studies, users and access rights - Creation of entry masks for standard and Web studies - CRF receipt tracking, double data entry, data entry tracking - Online CRF data entry - Data import based on ASCII files - Double data entry confrontation - Edit checks, creation and management of query forms - Data coding with the MEDDRA, WHODRUG, VEDDRA and ATCVET dictionaries - Data export to SAS, SPSS, EXCEL and to the ASCII, HTML and XML formats - Creation of reports, CRF, DHM... - Clinical trial monitoring - Security management, ORACLE database audit and backup - Creation and configuration of Patient Profiles - Utility allowing clinical studies to be imported and exported - Automatic update of the ORACLE database structure
a:1:{i:0;a:2:{s:4:"name";s:9:"Function:";s:3:"val";s:25:"clinical trial management";}}