Under the umbrella of the World Health Organization, its Emergency Committee and several thousands of scientists looked into the challenges related to COVID-19 vaccine safety, efficacy and access.
You might also like:COVID-19 Vaccine: From Ethics of Early Access to Payments for Risk
The meeting of the Emergency Committee (EC) convened on 14 January under the International Health Regulations (2005) (IHR) regarding the coronavirus disease (COVID-19) agreed that the COVID-19 pandemic continues to constitute a Public Health Emergency of International Concern (PHEIC). The disease was declared a PHEIC last January, and the EC took place for the sixth time since the start of the pandemic headed the WHO Director-General Dr Tedros Adhanom Ghebreyesus.
The EC members reviewed the new variants of SARS-CoV-2 and assessed the implications of vaccination and testing programmes for various fields including international travel.
WHO recognised the global risk level as very high due, in part, to recent reports of new SARS-CoV-2 variants. With this regard, genomic sequencing and sharing of data on emerging variants should be available globally, the experts stressed highlighting the need to create a standardised system for naming the new variants.
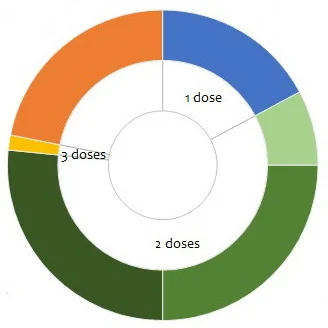
Dosage, schedule and route of administration of candidates in clinical phase (COVID-19 - Landscape of novel coronavirus candidate vaccine development worldwide, WHO)
Discussing the vaccine landscape and introduction, the need for equitable access through the COVAX Facility was emphasised. At the same time, the EC noted the delayed submission of vaccine data to WHO by some manufacturers, which inhibits the organisation’s ability to provide emergency use listing and ultimately affects equitable vaccine access. Technology transfer to low- and middle- income countries is also necessary to increase global production capacities, according to the EC.
The experts advised against the use of vaccination and testing certificates for international travel because “the impact of vaccines in reducing transmission is yet unknown, and the current availability of vaccines is too limited”. Instead, countries should focus on coordinated, time-limited, risk-based, and evidence-based approaches for health measures to ensure safe international travel, and cooperate to promote best practices.
On 15 January, WHO invited about 3,000 scientists from around the world to a virtual forum that focussed on identifying knowledge gaps and setting research priorities with regard to COVID-19 vaccines. Specifically, the participants discussed the safety and efficacy of existing vaccines and new candidates, potential optimisation of supply and the need for additional safety studies.
While acknowledging the unprecedented speed of the vaccine development and approval, “an astounding scientific accomplishment”, Dr Tedros noted that there was still a lot of work to be done. “More vaccines are in the pipeline, which must be evaluated to ensure we have enough doses to vaccinate everyone,” he said.
The participants discussed the issue of unequal access to vaccines globally. To date, 47 countries have started a vaccination programme having administered over 30 million doses of a vaccine; of those only one is a low-income country.
Similarly to the EC meeting the day before, the experts highlighted the need for further research on administering vaccines in different target populations, as well as on vaccination delivery strategies and schedules. Among others, the issues the vaccines’ efficacy and their impact on transmission rates were discussed together with the prospects of developing the next generation of vaccine platforms, ideally single-dose ones and with the possibility of scaling up the manufacturing.
In conclusion, the forum participants agreed on the establishment of a WHO-hosted platform for global sharing and coordination of emerging vaccine research information on efficacy and safety.
Source and image credit: WHO


![Tuberculosis Diagnostics: The Promise of [18F]FDT PET Imaging Tuberculosis Diagnostics: The Promise of [18F]FDT PET Imaging](https://res.cloudinary.com/healthmanagement-org/image/upload/c_thumb,f_auto,fl_lossy,h_184,q_90,w_500/v1721132076/cw/00127782_cw_image_wi_88cc5f34b1423cec414436d2748b40ce.webp)