HealthManagement, Volume 14, Issue 2/2012
Why are Clinicians using Biomarkers more Frequently?
The criteria to define the presence of sepsis (Levy et al. 2003) as well as to evaluate its clinical severity are not satisfactory since the signs and symptoms of sepsis are poorly specific and highly sensitive. Even more difficult than the diagnosis, is the monitoring of infection response to antibiotics (Povoa 2008). Currently the assessment of response relies on the resolution of the same criteria used in the diagnosis, however it may not be completely reliable as some clinical and radiologic variables can be influenced by non-infectious factors. Besides, the observation that a prompt and structured approach of severe sepsis and septic shock has a marked impact on prognosis encouraged the research on new tools of sepsis diagnosis even further (Marshall et al. 2009).
Since the inflammatory cascade plays a central role in the host-pathogen interaction and in the infection control mechanisms, these mediators have been successively assessed as potential biomarkers of infection. By definition, an ideal biomarker of infection should be absent if the patient is not infected, appear concomitantly and ideally precede the infection and disappear with successful therapy or remain elevated if infection is refractory to treatment (Povoa 2008). Whereas in myocardial infarction, 14 biomarkers are suitable for its diagnosis and prognostic assessment, in the complex field of sepsis more than 170 potential biomarkers have been studied and unfortunately the ultimate biomarker has not yet been identified (Pierrakos et al. 2010). What are the Questions we want to ask a Biomarker of Sepsis? Biomarkers are measures of molecular, biochemical or cellular levels that represent changes in the normal physiologic status. Biomarkers of sepsis indicate that the host has been exposed to an infectious pathogen, bacterial, fungal, viral or parasite, as well as the magnitude of the response to that infection. At the bedside, clinicians are faced daily with the two frequent dilemmas: (1) Whether a patient is infected or not and (2) If the response to antimicrobial therapy is adequate. In the presence of a patient with systemic inflammatory response syndrome (SIRS), particularly if associated with organ dysfunction, clinicians must consider the severity and site of the infection as well as the most probable agent and likely sensitivity patterns. In addition, clinicians need to monitor infection response to antibiotics as well as to ascertain the duration of antibiotic therapy, thus raising two additional questions:
• Is the infection refractory to ther apy? Should I change the antimi crobials?
• Is the infection cured? Can I safely stop antimicrobials?
Despite their importance, these questions are currently those most frequently cited by clinicians as 'impossible to answer with absolute confidence'. Biomarkers can be useful in some of these questions but the evaluation of their clinical performance is further complicated by the absence of a "gold standard" for the diagnosis of sepsis (Pierrakos et al. 2010).
What Questions Can Biomarkers Answer?
In the last 20 years, the research of biomarkers of sepsis has increased markedly. However, the great majority of studies evaluated their utility just in the assessment of prognosis. Biomarkers have a limited value if they are employed just to see if a patient has a high risk of dying when the attending physician is unable to change that prognosis. In opposition, we consider a biomarker useful if they provide additional information to a detailed clinical evaluation. In the context of infection and sepsis, biomarkers can potentially provide the following additional information:
- Screening;
- Diagnosis;
- Risk stratification;
- Monitoring response to therapy;and
- Antibiotic stewardship.
In this article we discuss recent data on the role of biomarkers of sepsis, in particular procalcitonin (PCT) and Creactive protein (CRP), in diagnosis and antibiotic stewardship.
Diagnosis
Both single as well as serial measurements of biomarkers have been evaluated in diagnosis, in a variety of infections as well as in clinical settings, namely emergency departments, medical and surgical wards and intensive care units (ICUs). However, the results are, at times, contradictory. This is a consequence of the choice of different methodologies, namely in inclusion and exclusion criteria, used for the selection of patients to be evaluated and analysed (Simon et al. 2004; Tang et al. 2007). In most studies, patients were included if they presented with SIRS and were subsequently stratified according to the American College of Chest Physicians/ Society of Critical Care Medicine (ACCP/SCCM) Consensus Conference criteria into sepsis, severe sepsis and septic shock (Levy et al. 2003). Such methodology could result in an assessment of clinical severity rather than the evaluation of the diagnostic accuracy of the biomarker in infection itself. The gold standard, which should be presence of documented infection, that is patients with a defined source of infection with positive cultures, as opposed to patients with no infection and no antibiotic therapy is frequently ignored (Cohen et al. 2001). Several studies have assessed the diagnostic performance of infection of a single measurement of a biomarker in different clinical settings and different infections (Table 1). In clinical practice, a markedly elevated serum level of a biomarker, e.g. CRP levels >5-10 mg/dL, may help to confirm the diagnosis of sepsis. Concerning PCT, the major limitation in diagnosis is the frequent finding of patients with documented infections with very low or even undetectable levels. This is particularly true in infections considered by the manufacturer to be localised, like empyema or abscesses (Christ-Crain et al. 2010).
At the bedside, clinicians should always consider the possibility of a falsepositive test because inflammatory stimuli other than bacterial infection can occur in critically ill patients, particularly during the first 72 hours of postoperative course and major trauma. Notwithstanding, usually these later conditions are usually easily diagnosed and identified as causes of biomarker elevations whereas changes in biomarker concentrations without an obvious reason can usually be caused by the emergence of infection and sepsis that are frequently silent in the beginning (Povoa 2008).
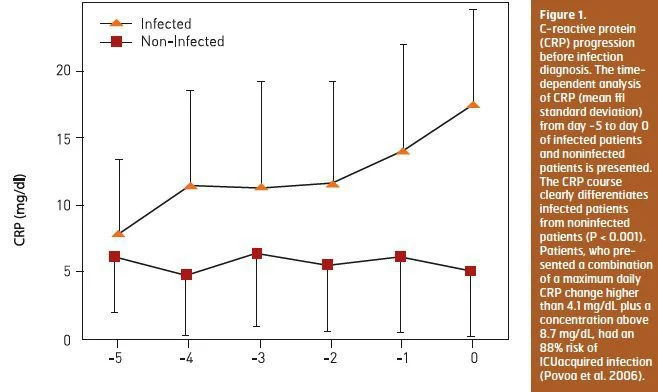
Since biomarkers are not static but on the opposite dynamic, with marked changes in serum concentrations over time, serial measurements could be more informative. Our group demonstrated that daily CRP determinations are useful as a marker of infection prediction in ICU patients admitted for longer than 72 hours. During the five days before the day of infection diagnosis CRP showed a steady and significant increase in infected patients, whereas in noninfected patients CRP remained almost unchanged (Povoa et al. 2006) (Figure 1). Patients, who presented a combination of a maximum daily CRP change higher than 4.1 mg/dL plus a concentration above 8.7 mg/dL, had an 88 percent risk of ICUacquired infection.
In a cohort of mechanically ventilated patients (Luyt et al. 2008), absolute PCT values as well as its kinetics over five days before clinical suspicion of pneumonia has been shown to have poor diagnostic accuracy for ventilator associated pneumonia (VAP) (AUC 0.51 and 0.62, respectively). More recently, two studies found that the diagnostic value of PCT to assess early onset pneumonia is poor in post-cardiac arrest hypothermia patients. In one study (Schuetz et al. 2010), PCT showed a steady decrease until day seven without differences in patients with and without presumed infection whereas CRP was significantly more elevated in patients with pneumonia.
Antibiotic Stewardship
The decision to start and stop antibiotics is probably one of the most frequent and difficult decisions at the bedside. In addition, the recommended durations of antibiotic therapy of the majority of infections are not based on data from randomised trials.
Two studies have demonstrated in VAP, that the implementation of a discontinuation antibiotic policy (Micek et al. 2004) as well as a fixed antibiotic duration (Chastre et al 2003) could significantly decrease the duration of antibiotic therapy to 6 and 8 days, respectively, in comparison to traditional and longer antibiotic durations of the control groups, 8 and 15 days, respectively, without any differences in outcome. It is important to emphasise that both studies were conducted without the use of biomarkers! Several original trials showed that the use of PCT in different infections, lower respiratory tract infection, acute exacerbation of chronic bronchitis, community-acquired pneumonia (Christ- Crain et al. 2006) and VAP (Stolz et al. 2009), could safely decrease the rate of antibiotic prescription and the duration antibiotic therapy. However, these analyses were markedly biased by the very long antibiotic therapies of the controls. In ProCAP (Christ-Crain et al. 2006), ProHOSP (Schuetz et al. 2009) and ProVAP (Stolz et al. 2009) trials, the control groups were on antibiotics for 12, 10 and 15 days, respectively!
In the ICU setting, several trials have been recently published assessing the role of PCT guided antibiotic therapy (Bouadma et al. 2010; Hochreiter et al. 2009; Jensen 2009; Nobre et al. 2008). With one exception (Jensen 2009), PCT-guided group showed a significantly lower duration of antibiotic therapy and smaller antibiotic exposure. However, there are several caveats in these studies that need to be discussed. In two trials (Hochreiter et al. 2009; Nobre et al. 2008), more than 70 percent of the eligible patients were excluded for reasons that were difficult to accept since they are common in an ICU setting, namely Pseudomonas aeruginosa infection. In the PRORATA trial, there were significant rates of protocol violations in the PCT-guided group (Bouadma et al. 2010). In 71.2 percent of the episodes of clinical decision, the attending physicians did not follow PCT-guided recommendations for several reasons. At inclusion, 69 infected patients had PCT<0.5μg/L, but in 94 percent the attending physician prescribed antibiotics against the recommendations. In follow-up, antibiotics were stopped in 39 pts with PCT>0.5μg/L, since they were considered clinically cured also against the recommendations; in 111 patients, antibiotics were maintained even after discharge (N=32) and in 79 unstable patients despite a PCT<0.5μg/L (Bouadma et al. 2010). Finally, two trials (Bouadma et al. 2010; Jensen et al. 2009) demonstrated that patients from the PCT-guided groups presented more organ dysfunction and failure, in particular late failure.
In a pragmatic, 2x2 factorial, cluster randomised trial in which two interventions were tested, availability of a CRP test and/or training in communication skills, clearly showed that availability of a CRP test could significantly decrease antibiotic prescription (Cals et al. 2009). This result is noteworthy since this study was performed in The Netherlands, which is the European country with the lowest antibiotic prescription in the community.
Conclusions
The ideal biomarker has not yet been identified. Unfortunately, multiple biomarkers correlate only with mortality and few add additional valuable information that can be useful in the clinical decision making process at the bedside. Among all known biomarkers probably PCT and CRP are those with more solid data. Is it time yet to use biomarkers in sepsis?
The answer is clearly yes but NEVER to be used solely, always in conjunction with a complete clinical evaluation and with a perfect knowledge of its biology, strengths and limitations.