Authors
Dr. Leo Lawler
Department of Radiology
Dr.Matt Crockett
Department of Radiology
Dr. Eoin Kavanagh
Department of Radiology
Prof.Sean Murphy
Stroke Physician
Mater Misericordiae University Hospital
Dublin, Ireland
“in the acute setting, deciding which patients should not proceed to interventional therapy is as important as deciding which patients should proceed”
Introduction
Until recently, the management of ischaemic stroke has largely been a passive process, centred on control of patient complications and rehabilitation of acquired deficits. However, recently there has been a refocusing of attention on more proactive stroke management, with interventional radiologists now often playing a central role within the stroke management pathway. Multiple therapeutic options are now available for managing acute ischaemic stroke. All of these therapies aim to achieve early recanalisation of occluded arteries leading to early vascular reperfusion of the ischaemic penumbra, the area of potentially salvageable brain tissue surrounding the permanently infarcted core. The currently available acute stroke therapies can be categorised according to the technique used to achieve reperfusion:
- Intravenous thrombolysis (IVT)
- Direct intra-arterial thrombolysis (IAT)
- Intra-arterial mechanical thrombectomy
Due to their heavy reliance on image guidance, the latter two therapies are generally performed by interventional radiologists, and form the main focus of this paper. However, the role of the interventional radiologist cannot be taken in isolation from the stroke care pathway, which is of the utmost importance.
Pre-Intervention Stroke Care
The stroke care pathway begins at the moment of the first clinical signs of stroke. It is often quoted that in the setting of stroke that ‘time is brain’; certainly if outcome is to be optimised a rapid recognition of symptoms and expedient transfer to an adequately equipped medical centre is essential. This allows not only emergency care to be administered, but also maximises the chance of successful interventional therapy. We need the right patient, in the right place, at the right time.
Image credit: American

Heart Association, American Stroke Association
The key stages in acute emergency stroke care are:
- Early recognition of stroke symptoms by patients, members of the general public or their physician in the community. Health information strategies have been key in this area with the ‘FAST’ public education campaign leading to a 55% increase in stroke-related emergency calls in the UK, for example.
- Rapid transfer to a hospital with the necessary expertise for multidisciplinary stroke care.
- Timely non-invasive imaging of the brain. This may include:
- Non-contrast brain imaging using CT or MRI;
- CT or catheter-directed angiography;
- CT/MRI perfusion/diffusion imaging;
- Carotid Doppler.
- Multidisciplinary triage to improve selection of patients suitable for more aggressive interventional therapy. In the acute setting, deciding which patients should not proceed to interventional therapy is as important as deciding which patients should proceed, in order to minimise complications and optimise outcomes.
- Initiation of appropriate intervention, be it thrombolysis or thrombectomy, without delay in those patients suitable for this therapy.
Imaging in Acute Ischaemic Stroke
Imaging plays a central and active role in the management of ischaemic stroke. Non-contrast CT and MRI are still the mainstays of acute stroke imaging. Other modalities include non-invasive CT/MR angiography, CT/MR perfusion and MR diffusion, which can give an imaging phenotype to the presentation. Invasive digital subtraction angiography may be used both in a diagnostic or therapeutic fashion. There is no universal agreement on one imaging algorithm and most imaging triage and protocols are dependent on local resources of people and hardware. The goals of imaging are to:
1.Confirm the diagnostic suspicion of stroke;
2.Characterise the stroke event and its distribution;
3.Guide candidature for various therapeutic interventions.
Presence of haemorrhage, intravascular thrombus and the relative size of core and penumbra are key features. Some also comment on collateral circulation and arterial and venous dynamic flow patterns. Most groups agree on the role of angiography - computed tomography angiography (CTA), magnetic resonance angiography (MRA) or digital subtraction angiography (DSA), but the role of perfusion imaging has waxed and waned as groups explore its predictive value. Although the ischaemic penumbra can be defined objectively using CT or MRI perfusion studies, its biologic correlate and link to therapy and outcome remains elusive. Some interventionalists prefer to transfer the patient to the angiography suite immediately, performing cone-beam angiography and bypassing traditional non-invasive imaging. At our institution patients undergo non-contrast CT brain followed by dual-phase CTA.
Intra-arterial Thrombolyis (IAT)
Catheter-directed delivery of fibrinolytic drugs directly to the site of thrombus in the cerebral circulation intuitively seems like a good idea. Early studies focused on delivery of IAT agents into the larger proximal vessels, the carotids and vertebrals. These studies demonstrated high rates of recanalisation (75-80%) (Casto et al. 1992; del Zoppo et al. 1988), but with significant levels of haemorrhagic transformation (between 20-30%) (Fitt et al. 1993).
Microcatheter design allowed more distal access into the thrombus and beyond, with the randomised PROACT I and PROACT II trials demonstrating high recanalisation rates of 66% (compared to 18% in the control group) (del Zoppo et al. 1998; Furlan et al. 1999). In spite of a higher rate of early intracranial haemorrhage in the IAT group (10% vs. 2% in the control), outcomes were significantly improved at 90 days with a 15% benefit in functional independence. A trial of IA thrombolysis in thrombotic middle cerebral artery (MCA) strokes reported higher rates of favourable outcomes in the IAT group compared to the IVT group with better recanalisation of arteries occluded by large thrombi (Mattle et al. 2008). A recent meta-analysis comparing IAT to ‘standard treatment’ or IVT published earlier this year suggested only a modest benefit for IAT over standard treatment, but no clear benefit of IAT over IVT (Nam et al. 2013). Small case series still suggest that IAT is a reasonably safe treatment option in certain patients in whom IVT is contraindicated.
Many early studies were undertaken using urokinase via the IV route, and there has been some extrapolation of this data to newer thrombolytic agents and to delivery of these agents via the IA route. Currently the most commonly used thrombolytic agent is acteplase, tissue plasminogen activator (tPA) produced using recombinant DNA technology.
Although thrombolytic treatment with tPA is of proven benefit, this agent is far from ideal, with other thrombotic agents, such as Tenecteplase, demonstrating superiority in some studies. Many other novel agents are also under investigation. Desmoteplase is a plasminogen activator, derived from the saliva of vampire bats, which has shown high rates of reperfusion when given in IV form (70% compared to 19% placebo) and low rates of intracerebral haemorrhage. Further trials (DIAS-3 and DIAS-4) are ongoing, and desmoteplase may well be suitable for targeted IAT therapy.
Other newer agents include fibrinogen-depleting substances derived from snake venom (alfimeprase, ancrod), which have demonstrated mixed results when used IV, and may be more effective via IA delivery. Active plasmin delivered directly to the site of thrombus via catheter and microplasmin are also under investigation. Lysis may be administered in association with instrumentation, but there are no set protocols in this regard.
Current recommendations from the American heart and stroke associations (Jauch et al. 2013) state that:
- IAT is a valid treatment option in patients with major ischaemic MCA strokes of < 6 hours’ duration who are not candidates for IVT or in large artery occlusion refractory to IVT;
- IAT should only be performed at an experienced stroke centre with access to suitable expertise.
It is noteworthy that fewer centres practise IAT with tPA and few perform IAT without thrombectomy, except perhaps in the posterior circulation where there are limited other options.
Mechanical Thrombectomy
The effectiveness of IAT for large clot burdens is questionable, and traditionally there was little beyond suction thrombectomy available to the interventionalist for clot retrieval. The last decade has seen the emergence of a series of devices designed specifically for intra-cranial clot retrieval.
The Merci clot retrieval system was the first system to obtain FDA approval in 2004, and helped generate interest in the area of clot retrieval. Memory-coiled nitonol loops delivered by microcatheter with balloon occlusion of the artery sought to remove the clot. The MERCI 1 trial showed recanalisation was achieved in 43% using the device alone and 64% using the Merci device with additional IAT (Gobin et al. 2004). The Multi Merci trial for a newer generation of Merci devices demonstrated a higher rate of recanalisation with newer devices, 57% with the device alone and 69% when used in conjunction with IAT (Smith et al. 2008). A recent review including over 1,200 patients treated with the Merci device concluded that it was a safe treatment modality for patients presenting with intracerebral large vessel occlusion within 8 hours of onset, though there is a lack of randomised clinical trials comparing the Merci system to IVT (Alshekhlee et al. 2012).
The Phenox clot retrieval system uses microfilaments to retrieve the clot. The most recent study assessed the device in a case series of 54 patients, and concluded that it was a safe and effective treatment option, with 54% recanalisation achieved with the Phenox alone and 72% recanalisation achieved when using the Phenox as part of a multimodal approach (Prothmann et al. 2012).
The Penumbra system adds vacuum to the process, and studies have shown that 81% arterial recanalisation was achieved with minimal adverse effects reported, with a good neurological outcome achieved in 57% (Clark et al. 2009). A recent meta-analysis of observational studies using the Penumbra system included over 400 patients, and demonstrated recanalisation rates of 86%,with average puncture to recanalisation time of 64 minutes (Almekhlafi et al. 2013).
Open stent design has been explored as a means to withdraw clot. Two examples include the Trevo and Solitaire. The TREVO 2 study demonstrated revascularisation rates of 86% and good clinical outcomes of 40% (Walcott et al. 2013). A recent review, published in 2013, analysed the cumulative human experience of the Trevo system, included 221 patients, and reported a revascularisation rate of 83% and a good outcome rate of 51% (Walcott et al. 2013). The SWIFT trial, a prospective, randomised, multicentre trial comparing the Solitaire device to the Merci clot retrieval system showed that significantly better revascularisation rates were achieved with the Solitaire device (61%) than the Merci device (24%), with corresponding 90-day good neurological outcome rates of 58% and 33% respectively (Saver et al. 2012). A recent publication of the North American Solitaire registry clinical outcome results included 354 patients compared favourably to the SWIFT trial with similar rates of recanalisation (83-87%) and a high 90-day good neurological outcome (Zaidat et al. 2013).
The experience in our jurisdiction would reflect AHA guidelines that favour stent-retrieval platforms over wire-loop systems. Though newer devices have improved the safety profile of thrombectomy, trial evidence has been challenged on methodological grounds. The ESCAPE trial seeks to directly measure the benefit of stent-retrieval versus best medical therapy (University of Calgary).
Many other experimental techniques are currently under investigation, all of which aim to achieve rapid recanalisation and reperfusion of the ischaemic penumbra in acute stroke. These include intracranial stenting, balloon pump technology, flow reversal strategies and ultrasound-accelerated thrombolysis.
Conclusion
The era of the pro-active management of stroke patients has arrived and is here to stay. As we progress, we learn how much we still do not know. There is currently no accurate imaging biomarker to determine which patients will benefit most from which intervention. Existing limits in thrombolytics and devices are being addressed with rapidly evolving translational and clinical research. These efforts are reflected in the rapidly changing patient algorithms. Imaging developments will help refine pathways for individualised care and help marry specific pharmacological-thrombectomy combinations to those who will benefit most with the least risk of adverse effects. Much done, much more to do and continued critical reflection on our processes will be key.
Images
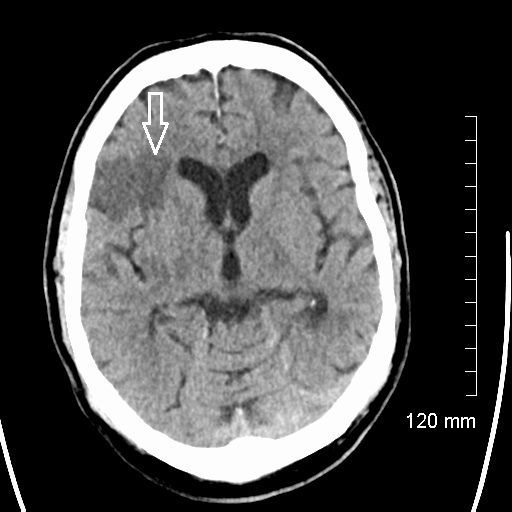
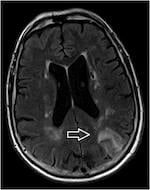
1.Noncontrast axial CT of acute right MCA infarct.
2.
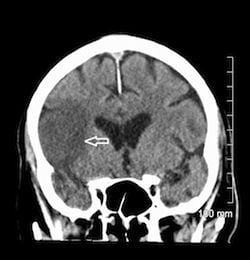
Noncontrast coronal CT of acute right MCA infarct.
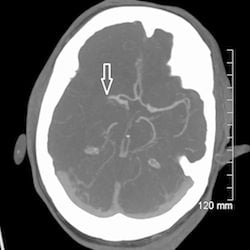
3. CT cerebral angiogram MIP axial image of occlusive right MCA thrombus
 .
.4.
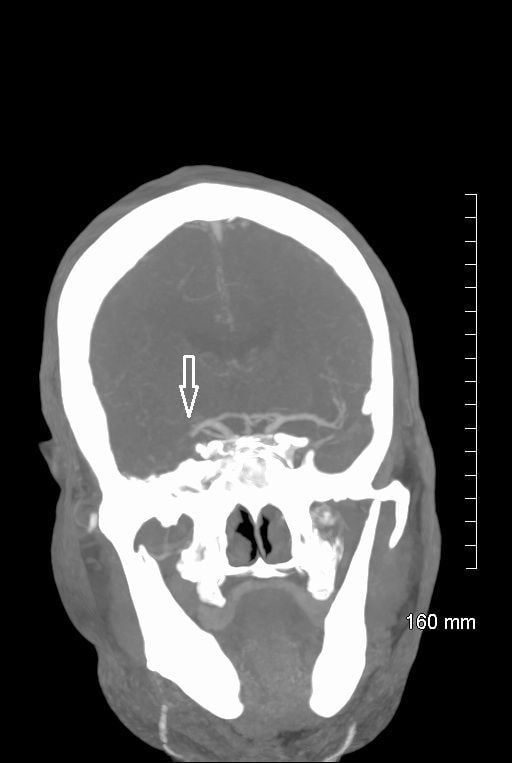
CT cerebral angiogram MIP coronal image of occlusive right MCA thrombus.
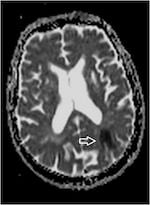
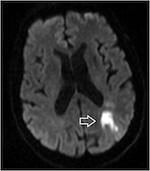
5.6.MRI acute left occipital infarct ADC map MRI acute left occipital infarct diffusion weighted sequence (below, left)
7.
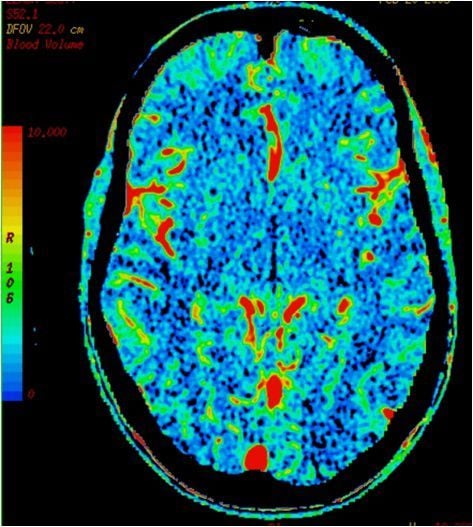
8.CT perfusion right MCA infarct - cerebral blood volume (left, above)
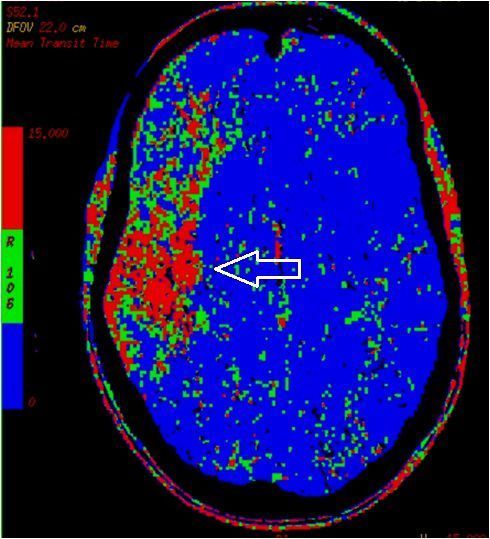
9.CT perfusion right MCA infarct - mean transit time (middle, above)
10.CT perfusion right MCA infarct - cerebral blood flow (right, above)
MRI acute left occipital infarct T2 FLAIR sequence
Almekhlafi MA, Menon BK, Freiheit EA et al. (2013) A meta-analysis of observational intra-arterial stroke therapy studies using the Merci device, Penumbra system, and retrievable stents. AJNR Am J Neuroradiol, 34(1): 140-5.
Alshekhlee A, Pandya DJ, English J et al. (2012) Merci mechanical thrombectomy retriever for acute ischemic stroke therapy: literature review. Neurology, 25;79(13 Suppl 1): S126-34.
Casto L, Moschin L, Camerlingo M et al. (1992) Local intraarterial thrombolysis for acute stroke in the carotid artery territories. Acta Neurol Scand, 86(3): 308-11.
Clark W, Lutsep H, Barnwell S et al. (2009) The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke, 40(8): 2761-8.
del Zoppo GJ, Ferbert A, Otis S et al. (1988) Local intra-arterial fibrinolytic therapy in acute carotid territory stroke: A pilot study. Stroke, 19(3): 307-13.
del Zoppo GJ, Higashida RT, Furlan AJ et al. (1998) PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke, 29(1): 4-11.
Fitt GJ, Farrar J, Baird AE et al. (1993) Intra-arterial streptokinase in acute ischaemic stroke. A pilot study. Med J Aust, 159(5): 331-4.
Furlan A, Higashida R, Wechsler L et al. (1999) Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA, 282(21): 2003-11.
Gobin YP, Starkman S, Duckwiler GR et al. (2004) MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke, 35(12): 2848-54.
Jauch EC, Saver JL, Adams HP Jr et al. (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 44(3): 870-947
.
Mattle HP, Arnold M, Georgiadis D et al. (2008) Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke, 39(2): 379-83.
Nam J, Jing H, O'Reilly D (2013) Intra-arterial thrombolysis vs. standard treatment or intravenous thrombolysis in adults with acute ischemic stroke: a systematic review and meta-analysis. Int J Stroke, Jan 7. doi: 10.1111/j.1747-4949.2012.00914. [Epub ahead of print]
Prothmann S, Lockau H, Dorn F et al. (2012) The phenox clot retriever as part of a multimodal mechanical thrombectomy approach in acute ischemic stroke: single center experience in 56 patients. ScientificWorldJournal Apr 24: 190763. doi: 10.1100/2012/190763. [Epub]
Saver JL, Jahan R, Levy EI et al.(2012) Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet, 380(9849): 1241-9.
Smith WS, Sung G, Saver J et al. (2008) Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke, 39(4): 1205-12.
University of Calgary. Endovascular treatment for small core and proximal occlusion ischemic stroke (ESCAPE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [cited 2013 November 5] Available at: http://clinicaltrials.gov/ct2/show/NCT01778335
Walcott BP, Boehm KM, Stapleton CJ et al. (2013) Retrievable stent thrombectomy in the treatment of acute ischemic stroke: Analysis of a revolutionizing treatment technique. J Clin Neurosci, 20(10): 1346-9.
Zaidat OO, Castonguay AC, Gupta R et al. (2013) North American Solitaire Stent Retriever Acute Stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg, Sep 23. doi: 10.1136/neurintsurg-2013-010895. [Epub ahead of print]
 Heart Association, American Stroke Association
Heart Association, American Stroke Association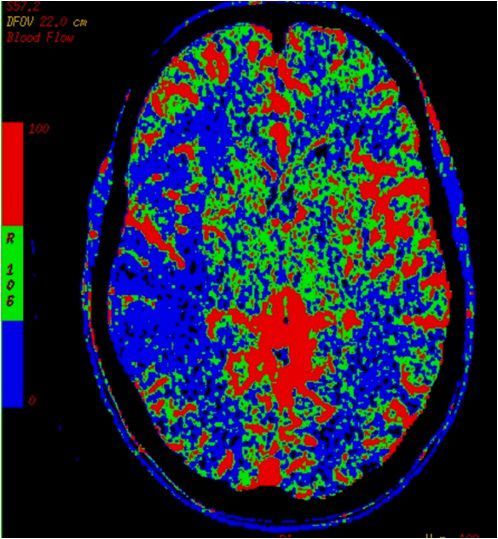
 Noncontrast coronal CT of acute right MCA infarct.
Noncontrast coronal CT of acute right MCA infarct. .
. CT cerebral angiogram MIP coronal image of occlusive right MCA thrombus.
CT cerebral angiogram MIP coronal image of occlusive right MCA thrombus.