HealthManagement, Volume 21 - Issue 2, 2021
Parenchyma-sparing liver surgery (PSLS) is becoming increasingly important as a salvage surgical therapy for patients with hepatic metastases from colorectal cancer. We report our experience with a medical app that allows to personalise a digital 3D model of the liver on which radiologists manually draw hepatic lesions as if using a digital “notepad”, thus summarising the full preoperative CT and/or MRI datasets that are stored in the picture archiving and communication system (PACS). The app provides an ideal link between preoperative imaging and liver surgery, since it simplifies surgical planning and it assists PSLS by providing quick reference in the operating theatre. Moreover, this app can be used in training as an effective educational tool.
Key Points
- Parenchyma-sparing liver surgery (PSLS) is an important salvage surgical therapy for patients with hepatic metastases from colorectal cancer.
- Using the principles of PSLS, hepatectomies can be performed with specific indicators of the tumour position and vessels to resect.
- In Pisa, a clinical and technical multi professional team developed a medical app that allows radiologists to personalise an electronic 3D model of the patient’s liver.
- Graphically simplifying the complex radiological information into a patient personalised 3D liver model has the potential to improve planning and performing challenging procedures such as PSLS.
Introduction
Parenchyma-sparing liver surgery (PSLS) is a highly sophisticated surgical approach which is becoming increasingly important as a salvage surgical therapy for patients with hepatic metastases from colorectal cancer. It allows a small volume of resection (less than three adjacent segments) with the goal of ensuring an adequate volume of the liver remnant and an adequate vascular outflow (Urbani et al. 2017).
This surgical procedure overcomes the concept of traditional surgical subdivision of the liver along longitudinal lines, going beyond the Brisbane 2000 Nomenclature (Strasberg and Phillips 2013). Using the principles of PSLS, hepatectomies can be performed with specific indicators of the tumour position and vessels to resect (Torzilli et al. 2017).
However, since PSLS is very difficult to standardise, surgeons often feel the need to have a comprehensive albeit simple visual representation of the liver with the exact position of each metastatic lesion to resect, together with the pertinent vascular information.
The 2D and 3D radiological images routinely provided by CT and/or MRI may fail to adequately meet the surgeon’s needs. In fact, a relatively simpler three-dimensional model that graphically summarises the complete radiological data-sets provided by CT and/or MRI is easier to be used in surgical planning as well as a quick reference in the operating theatre.
In Pisa, a clinical and technical multi professional team developed a medical app that allows radiologists to personalise an electronic 3D model of the patient’s liver - using the whole imaging information stored in the picture archiving and communication system (PACS) - by manually drawing and placing the hepatic lesions into the model liver as if using a digital “notepad”.
We report our experience in using the medical app for planning and intraoperative guidance in PSLS and also as an educational tool for surgeons in training as well for other junior doctors and students.
The App
A multidisciplinary group of radiologists and surgeons collaborated with software developers to create and progressively improve a medical app that has been developed for Android and iOS, and a software for Mac OS and Windows. It mainly consists of:
• an editable 3D model of the liver realised with Unity3D;
• a rendering engine which makes it possible to browse through the model and move the lesions;
• a system for managing and archiving patient data, and for statistical compilation.
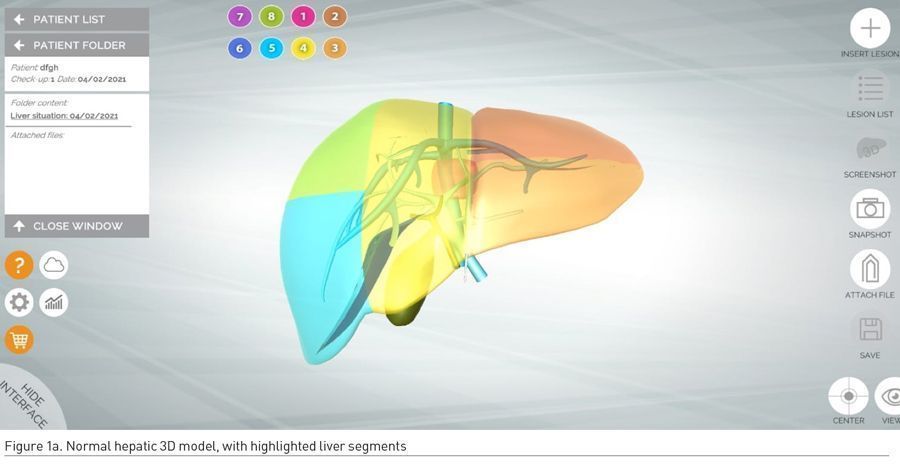
The default liver model has a standard map of venous vessels (venous portal system and hepatic veins) and a schematic biliary system (common bile duct, right and left hepatic ducts and gallbladder). In this digital 3D model of the liver the eight segments - according to the classification of Couinaud - are displayed in different colours (Figure 1a).
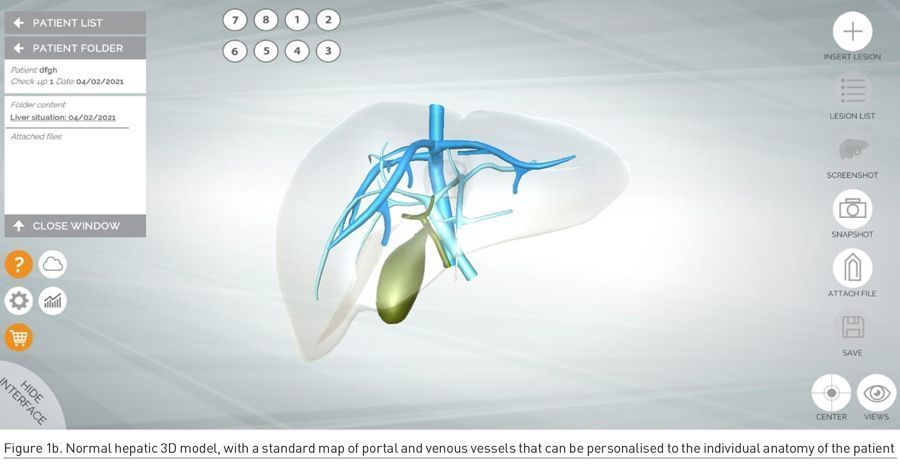
In order to accommodate individual variations of the hepatic vascular architecture, the app allows to insert intrahepatic communicating veins or accessory hepatic veins, thus achieving personalisation of the liver model (Figure 1b). This is extremely useful for the exact positioning of the lesions within the liver and for predicting post-surgical venous outflow.
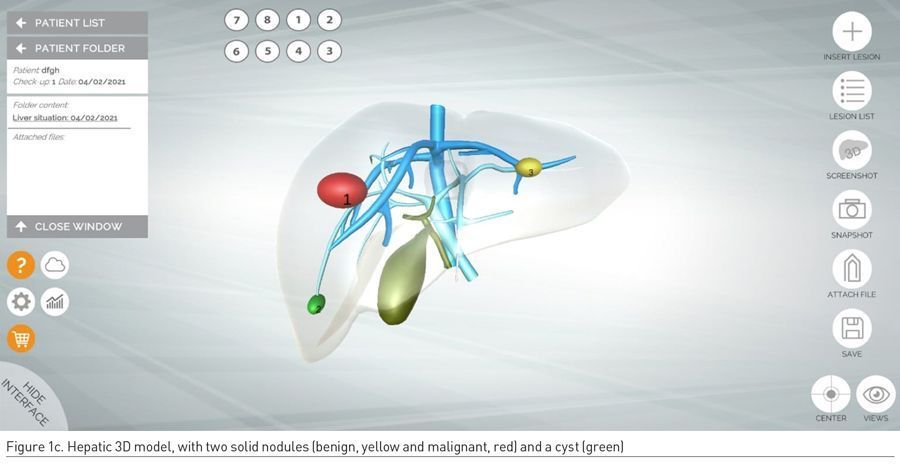
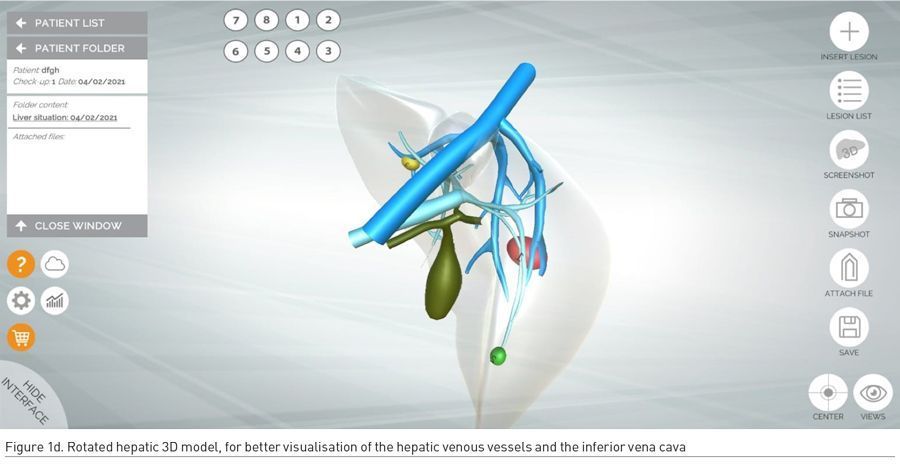
Once the 3D model of the liver and its hepatic outflow has been personalised to the individual anatomy of the patient, lesions are manually added by radiologists on the basis of preoperative CT and/or MRI datasets. The following colour code is used: red lesions are malignant, green lesions are simple cysts, whereas yellow lesions are solid benign hepatic lesions such as focal nodular hyperplasia, nodular regenerative hyperplasia, haemangioma, and adenoma (Figure 1c-d).
During the clinical workflow, all medical members of the multidisciplinary team involved in the oncologic management of patients with colorectal cancer liver metastases can consult and modify the notes wherever they are (even offline). All the app information is safely stored on a cloud server. A reliable storage of the information is ensured by iCloud.
Our Experience
Over the past three years, we have used this app in the clinical management of patients eligible for PSLS as well as in teaching applications. Concerning the latter, the app was tested as a teaching tool for residents, fellows and surgeons in PSLS training.
Concerning clinical applications, we noticed that graphically simplifying the complex radiological information into a patient personalised 3D liver model has the potential to improve planning and performing challenging procedures such as PSLS.
In fact, the app shows the hepatic lesions in an easy and intuitive way, using display devices that can be introduced into operating rooms without disrupting surgical workflow. It allows surgeons to focus on findings that are of key importance in designing liver cut surfaces and to simulate surgical steps. Moreover, the app may help elucidating complex cases involving several lesions or lesions close to blood vessels.
Another useful feature of the app is its immediacy for all users, including patients. In our experience, this app facilitated communication between healthcare personnel and patients, particularly when it came to obtaining informed consent. The 3D model intuitively shows to the patients their “hepatic situation”, making it easier to explain surgical options as well as to justify the need for a lengthy procedure and the possibility of complications.
We observed that informed consent obtained after showing this cartoon-like 3D model was associated with a lower level of patient anxiety in comparison to the consent obtained by showing actual radiological images which are often perceived as incomprehensible and menacing.
A potential weakness of the app is the amount of time that radiologists have to spend to summarise the whole information contained in the preoperative CT and/or MRI datasets (consisting of thousands of images) into the 3D model containing just the key information required by the surgical team. However, such investment of radiologists’ time is compensated by the reduced request of clinico-radiological consultation in the different phases before and after surgery. Moreover, the personalised 3D model of the metastatic involvement of the liver can be used in communicating with patients as well as in training applications.
Conclusions
Our preliminary experience showed the added value of the efficient sharing of 3D liver models that was made possible by this user-friendly platform which can be run on devices that are increasingly integrated with the daily activities of medical professionals.
With the use of this app, it is possible to support the training of surgeons in planning and performing PSLS. Furthermore, this medical app can be an important educational tool for many trainees since it can be utilised by radiology residents as a test for positioning hepatic lesions on the basis of CT and/or MRI datasets and by surgery residents for simulating cut surfaces resection on the liver model. A slightly modified version of the app was even used to improve teaching the CT anatomy of the liver to medical students.
Conflict of Interest
None. 
References:
Strasberg SM, Phillips C (2013) Use and Dissemination of the Brisbane 2000 Nomenclature of Liver Anatomy and Resections Ann Surg., 257(3):377-82.
Torzilli G, Viganò L, Gatti A et al. (2017) Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB (Oxford), 19(9):775.
Urbani L, Colombatto P, Balestri R et al. (2017) Techniques of parenchyma-sparing hepatectomy for the treatment of tumors involving the hepatocaval confluence: A reliable way to assure an adequate future liver remnant volume. Surgery, 162(3):483-499.




