HealthManagement, Volume 19 - Issue 2, 2019
Quality Assurance as a function of healthcare business profitability and efficiency
Healthcare needs to invest in quality assurance and improvement to use resources and increase profitability.
Quality Assurance (QA) is a programme for the systematic monitoring and evaluation of the various aspects of a project, service, or facility to ensure that standards of quality are being met. QA is a wide-ranging concept covering all matters that individually or collectively influence the quality of a product. Simply put, QA involves all activities geared towards the maintenance of a desired level of quality in a service or product (who.int).
QA used to be relevant mainly in the health sector, specifically the pharmaceutical industry, but the term is now applicable to all industry sectors involving any type of service or product such as medical and electronic devices, software and hardware in information technology industry. This expansion of the number of industrial sectors to assure their products and services are of good quality, has also necessitated the development of international regulations to drive and assure compliance to quality claims by these industries. Some of these regulations implemented as norms, standards and guidelines to promote QA are enforced by different regulating bodies such as World Health Organization (WHO), International Standards Organisation (ISO), International Electrotechnical Commission (IEC), different National Regulatory Agencies (NRAs) and the list goes on as more industrial sectors imbibe the quality principles.
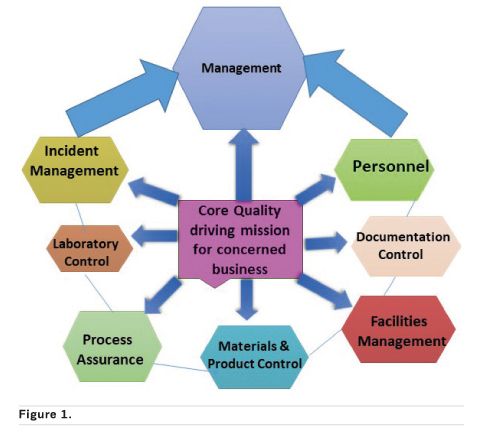
The basic principle connecting all these different quality assessments, is the Quality Management System (QMS) principle depicted in Figure 1 below as the quality management framework components.
Some businesses simply divide QA into four major areas: quality control, production, distribution, and inspections. Irrespective of how a company views quality it must be handled in an organisation as a top-down process with the company’s management team making it a core part of its mission. If the management’s view about QA is that it’s bureaucratic, a waste of time and not necessary to meet the financial bottom line, personnel will toe the same line and consequently impact other processes. The core of this article is to emphasise how paying close attention to QA from start to finish of a product’s or service’s lifecycle can impact an organisation’s profitability. QA or quality in general has indicators that are measurable, objective, quantitative measures of key system elements of performance, using this simple process in Figure 2 to drive compliance.

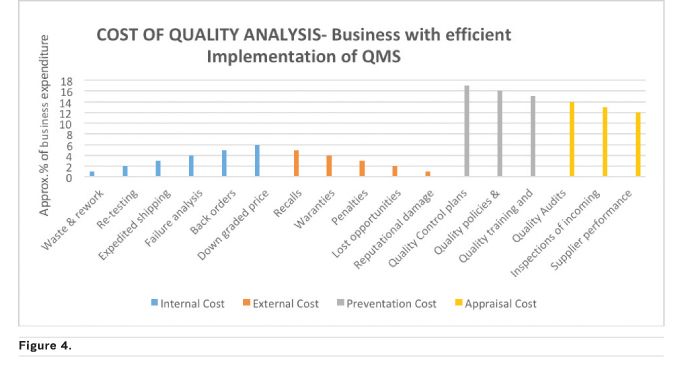
In healthcare, more time should be invested in planning for quality, followed by implementing the QMS principles, continuous monitoring for improvement and acting on the improvement initiatives in a timely manner to yield needed outcomes including increase financial benefits. The increase in business profitability can better be explained by showing different scenarios using some of the Cost of Quality (COQ) (Accounting for Management no date) elements as shown in Figures 3 and 4 below. COQ analysis concerns the business expense involved in preventing/controlling, detecting/monitoring and removing/addressing defects or quality incidences that affect a product or service. It is classified into four costing elements of Prevention Cost (planning phase), Appraisal/Inspections Cost (doing and checking), Internal Cost (correcting defects that happen internally) and External Cost (correcting defects that occur outside the organisation, after a product/service is out of the company). The external cost is the most damaging to a company both financially and reputational.
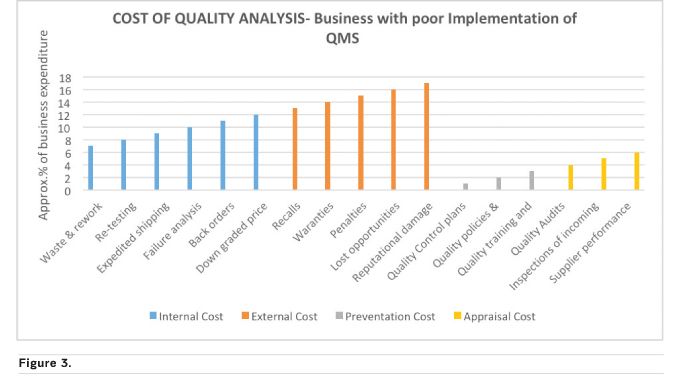
Figure 4 depicts a company that invests more and has a culture of thinking QA at the beginning, middle and end rather than a sudden remembrance to implement QA at the middle or end of a project or process. Figure 3 is almost the exact opposite, a company that does not put QMS at the core of everything they do with the ultimate price of spending a high percentage of their profit on cleaning up their reputation after the negative effect of a poor quality product gone into the market space. The scenarios above (common in manufacturing and distribution companies) can be applied to any type of company including healthcare companies using their applicable quality indicators. Examples of possible hospital indicators are Inpatient Quality Indicators (IQIs) and Patient Safety Indicators (PSIs).
Application of quality assurance in healthcare industry
The use of QA healthcare is becoming top priority for healthcare providers. Use of quality indicators measures and reports has helped to show that up to 98,000 deaths per year occurred in the United States (US) because of medical errors, thereby being among the top 10 causes of deaths (Jonge et al. 2011). The use of quality indicators to drive patient satisfaction and patronage has been in existence for a while; Health Canada, for example, has been providing health indicators reports since 2002 which contain information on healthcare across the country allowing governments and Canadians to compare data, track changes, see progress and identify areas for improvement within the healthcare system (Government of Canada 2017). This type of practice depicts the plan-do-check-act quality process which ultimately always leads to process improvement and profitability for any institution.
You might also like:Strategic product approval for for health companies and regulators
QA can be used to drive patients’ satisfaction and patronage in a healthcare setting as only a satisfied patient will be willing to pay for healthcare services. An example of this was well researched in developing communities (Brown et al. 1998). It states that QA promotes confidence, improves communication, and fosters a clearer understanding of community needs and expectations. If providers do not offer quality services, they will fail to earn the population’s trust, and clients will turn to the health system only when in dire need of curative care. This scenario is particularly unfortunate in developing countries, where the success of lifesaving preventive care, such as immunisation, growth monitoring, family planning, and antenatal care, depends on the willing participation of communities. Moreover, as primary healthcare programmes adopt cost-recovery strategies, the quality of service must be sufficient to attract the population to the clinic on a fee-for-service basis.
Recent trend is the use of Artificial Intelligence (AI) technology in healthcare such as in radiological imaging for the diagnosis of chronic diseases such as in cardiology and tissue perfusion.
Health companies manufacturing medical devices need to put a robust QA process in place that validates the use of these medical devices and addresses any potential risk. The importance of this aspect of the QA process is so urgent that the International Medical Device Regulatory Forum (IMDRF) in their fourth issue, provided a path for global regulators to converge on terminology, a risk-based framework, and an understanding of quality management system principles (U.S. Food and Drug Administration 2018). Hospitals and clinics using these devices must understand them, put their own quality tracking matrix in place to checkmate the manufacturers’ claimed device performance and know at what out of specification performance trend stage they must raise an alert to the Regulators and manufacturers. This will help protect the patients, health institution’s and its healthcare providers’ reputation. One of such QA processes to be put in place is a schedule of tracking the medical device maintenance to ensure optimal device output always. For example a simple medical device such as a blood pressure equipment can malfunction due to poor battery life or other internal equipment issues which could have been detected during routine maintenance, may lead to incorrect patient blood pressure readings and ultimately stroke, if true readings are high (200/160) but device shows readings are within acceptable limit (103/76).
Businesses including healthcare companies interested in profiting and driving efficiency in their products, processes and patient satisfaction, must make QA a way of living (culture) and not just a necessity because regulations demand it.
Key Points
- Organisations must put QA at the centre of their operations
- A culture of QA starts with top management
- There are internal and external costs, the latter including financial and reputational tolls
- QA can drive patient satisfaction and loyalty
References:
Brown LD et al. (1998) Quality Assurance Methodology Refinement Series - Quality Assurance of Health Care In Developing Countries. Bethseda: Quality Assurance Project.
Costs of quality or quality costs (nd). Accounting for Management. Available from accountingformanagement.org/costs-of-quality-or-quality-costs/
Essential medicines and health products (nd). WHO. Available from who.int/medicines/areas/quality_safety/quality_assurance/about/en/
Health Indicators (2011) Government of Canada. Available from canada.ca/en/health-canada/services/health-care-system/health-indicators.html
Jonge V et al. (2011) Overview of the quality assurance movement in healthcare. Best Practice & Research Clinical Gastroenterology 25/3:337-347
Software as a Medical Device (SAMD) (2018) U.S. Food and Drug Administration. Available from fda.gov/medicaldevices/digitalhealth/softwareasamedicaldevice/default.htm



