HealthManagement, Volume 17 - Issue 2, 2017
Computerisation of data collection in a multicentre clinical trial allows fast and easy conduct of the study. Cloud computing is a viable solution for storing patient data in virtual archives accessible by different hospitals. The adoption of an IT platform for centralised review of images and clinical reports in trials saves resources and time.
Multicentre clinical trials allow rapid collection of data involving great numbers of patients, and let researchers gain specialist advice from international experts. In multicentre clinical trials it is common practice to have clinical auditors at a central radiology centre who perform secondary reading of patient data produced by various radiological centres. CDs or DVDs of radiological images and paper documents with tumour size measurements, according to the Response Evaluation Criteria in Solid Tumours (RE CIST ) Image Transmittal Form (IT F) (Eisenhauer et al. 2009), are both sent by courier to the central radiology centre for secondary reading. This process is time- and resource-intensive, as it requires individual documents to be physically sent. As the number of patients needing review grows, the burden and delay increases
In order to improve the process, the research team at the radiology department in the University of Pisa hospital has developed an IT platform built on a cloudbased virtual server for a more rapid secondary review of images from multi-centre locations.
Cloud Computing
In oncological trials periodic follow-ups by means of CT or MRI are performed. These imaging modalities require substantial digital storage. Although the number of patients in the clinical trial is defined, it is not possible to determine in advance the total number of followup procedures that each patient may undergo; consequently the overall storage requirement cannot be predicted. In this context, the adoption of cloud technology has the benefit of being able to adapt dynamically to the needs of the trial in terms of storage.
Traditionally, a local server was employed for the management of centralised reading. This method has several preliminary requirements:
- Establish the extent of storage resources
- Acquire computing resources (CPU, RA M) suitable for replying to requests within a reasonable time
- Provide backup and recovery mechanisms in the event of failure
- Dedicated human resources for management of the system at low-level (hardware, operating system, networking, backup), intermediate level (applications and archives management, interactions between autonomous systems, security) and high level (use of applications and production tools for interpretation and processing of information)
As opposed to the traditional approach, the proposed cloud technology allows, depending on requirements, for more adaptive storage resources, and it does not require the use of dedicated human resources for the management of the physical system, as the cloud service provider undertakes this task.
IT Platform
The main issues that need to be considered in designing a platform for a fully electronic central review system are:
- The choice of a communication protocol for uploading images on the platform. The most frequently used protocols are File Transfer Protocol (FTP ) and Hypertext Transfer Protocol (HTTP )
- The choice of a Picture Archiving and Communication System (PA CS) for images storage
- Choice of a Digital Imaging and Communications in Medicine (DI COM) viewer
- Implementation of an electronic ITF
- Implementation of security measures to ensure compliance with U.S. Food and Drug Association (FDA) 21 Code of Federal Regulations (CFR) Part 11 on electronic records.
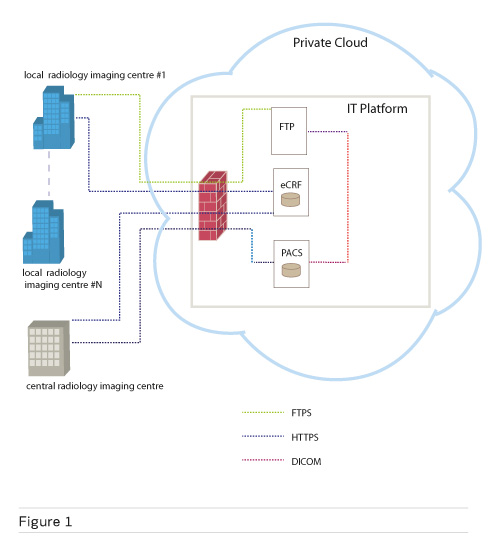
Figure 1 shows the IT platform and its main components. The platform has been installed on a virtual server within a private infrastructure-as-a-service (IaaS) cloud provided by the IT centre of the University of Pisa. The cloud-based virtual server runs the Ubuntu 14.04 operating system and is equipped with 8 CPU, 16 GB of RA M and a total of 500 GB of storage.
See Also: mHealth Trial Cuts Costs, Improves Access
Communication protocol for images upload
Images are uploaded to the IT platform using File Transfer Protocol (FTP ) protocol, then sent to the Picture Archive and Communication System (PA CS) through DI COM C-STORE transactions. Prior to uploading, each radiology imaging centre must perform anonymisation, de-identification and assignment of a patient identifier according to how it is specified in the trial protocol. De-identification and anonymisation are common strategies that are used to remove patient identifiers in electronic health record data. The use of these strategies in multicentre research studies is of paramount importance, given the need to share electronic health record data across multiple environments and institutions while safeguarding patient privacy.
PACS and DICOM viewer
The Dcm4chee open source PACS, backed by a MySQL database, is used for image archiving, and the web DICOM viewer, ‘Oviyam’ is used for image viewing. All communications between the viewer and PACS are done through the Web Access to Dicom Objects (WADO ) service.
Electronic ITF
The ITF is a web application that allows electronic management of all clinical information needed for centralised reading. The server side of the application is implemented in Java Enterprise Edition (EE), which leverages the GlassFish application server and can be used on any operating system with Java. The database server used by the application for data storage is the MySQL database engine, which provides different privilege levels to users based on their roles in the clinical trial. Local radiology imaging centres can upload and store measurements according to RE CIST criteria for each examination uploaded on the platform. The central radiology imaging centre can view the RE CIST measurements provided by local centres and submit the results of the review. The clinical trial sponsor can view all data stored on IT F, but cannot make any database changes. A system administrator creates users and provides them with appropriate privileges. In order to ensure compliance with FDA rule 21 CFR Part 11 on electronic records, the application provides an audit trail by recording every change to the database, not only the user who made the change, but also the time and date of modification.
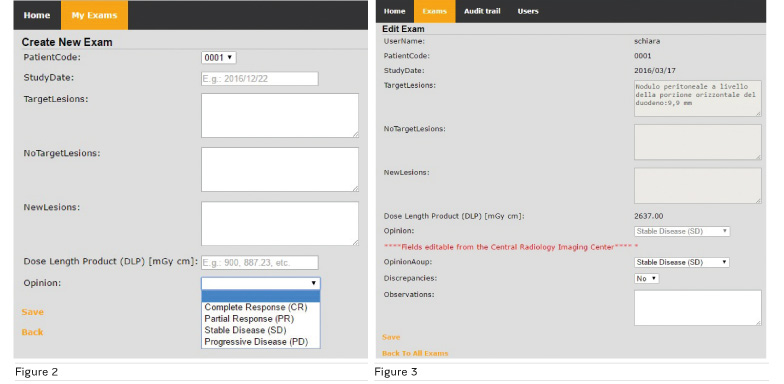
Figure 2 shows the form that local radiology imaging centres fill out to submit an imaging study for centralised reading. It includes information about the patient’s identifier, study date, measurements of lesions (target, non-target lesions and new), Dose Length Product (DLP) and global assessment of disease according to RE CIST criteria; Complete response, Partial response, Stable disease and Progressive disease.
Figure 3 shows
the form used by the central radiology imaging centre to report the results of
the review. It is in two parts. The first part is not editable and contains
information provided by the local radiology imaging centre that has submitted
the exam to centralised reading. The second part is used to archive the results
of the review, including the global assessment of the disease discrepancies
with the assessment provided by the local radiology imaging centre, and other
comments.
The cloud-based IT platform has been used for the management of the radiological images and their centralised reading in the clinical trial ERbitux MEtastatic colorectal cancer Strategy (ERMES), which was sponsored by Policlinico Agostino Gemelli (clinicaltrials.gov/ct2/show/NCT02484833). The University of Pisa Hospital plays a dual role, being both a local radiology imaging centre and the central radiology imaging centre.
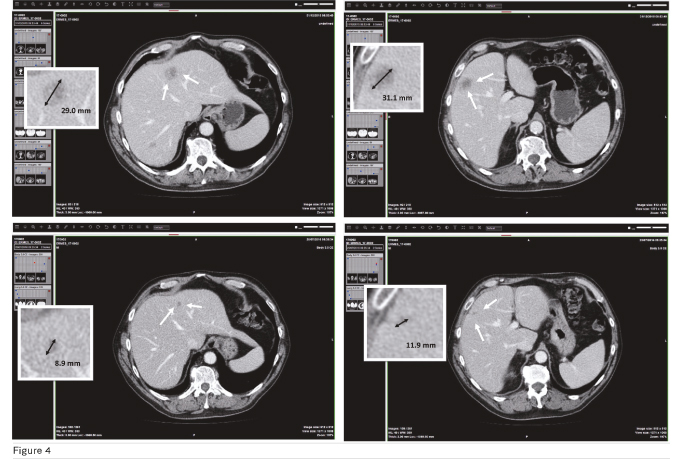
The clinical trial ERMES is a multicentric, non-inferiority phase III randomised study in patients with RAS and BRAF WT metastatic colorectal cancer. They are randomised to receive first line FOLFIRI + Cetuximab until disease progression (or unacceptable toxicity), or FOLFIRI + Cetuximab for 8 cycles followed by Cetuximab alone until disease progression (or unacceptable toxicity). Planned accrual time is 50 months, initially planned to be 24 months. In total 600 patients will be randomised, 300 for each arm. The end of the trial will be the date of the last visit of the last participant in the protocol. Final analysis of the data and report writing will occur after formal declaration of the end of the trial. As a participating centre, the University of Pisa has enrolled and randomised 3 patients; only one of them is still on treatment. The others have reached progression and thus are continuing with a second-line therapy. Among the 3 patients from Pisa, the patient who best represents the typical disease progression is patient number 17-0002 who was randomised on 21/01/2016, underwent first dose of therapy on 22/01/2016 and exited the trial on 23/12/2016. Disease assessment according to RECIST 1.1 shows two target lesions and a single non-target lesion in hepatic subsegments IV , V, VII , respectively. Figure 4 shows that the patient achieved a 65% decrease in tumour burden after 7 months of therapy, which according to RECIST 1.1 is considered a partial response.
Security
Security is ensured through the use of user names and passwords to uniquely authenticate users and provide different privilege levels to access the IT F. A local radiology imaging centre can only create, view and edit their own data and not those of other centres. The central radiology imaging centre can display all data and insert results of the review. The sponsor can display all data, but cannot make changes to the database.
Data exchange on the internet takes place over Secure Sockets Layer (SS L) encrypted channel. This happens for all FTP and HTTP connections. Firewall access to the DI COM viewer and PA CS is only permitted to users of the central radiology imaging centre.
Conclusion
Adoption of cloud technology for management of centralised reading in a cancer clinical trial has shown crucial advantages. Since the management of infrastructure is provided by the cloud service provider, it has allowed radiology department technical staff to exclusively devote their time to implementation of the IT platform. We have used the platform in clinical practice and verified its simplicity of use, both from the point of view of the local radiology imaging centre as uploaders of data, as well as that of the central radiology imaging centre as reviewers. Overall we have found that the platform allows prompt data availability, easy image comparison, fewer human errors and time and cost reduction, thus ensuring a more rapid secondary review.
Key Points
- Computerisation of data collection in a multi-centre
clinical trial allows fast and easy conduct of the study
- Cloud computing is a viable solution for storing patient
data in virtual archives that are accessible by different hospitals
- The adoption of an IT platform for the centralised review of
images and clinical reports in cancer clinical trials results in an overall
reduction of resources and time
References:
Eisenhauer EA, Therasse P, Bogaerts J et al. (2009). New response evaluation in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer, 45(2): 228-47.
European Medicines Agency (2002) ICH Harmonised tripartite guideline E6: note for guidance on good clinical practice (PMP/ICH/135/95). London: European Medicines Agency.
United States. Food and Drug Administration (2010) Code of Federal Regulations Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter A. General. Part 11. Electronic records; Electronic signatures. 21 CFR 11. [Accessed: 7 April 2017] Available from accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11






