HealthManagement, Volume 16 - Issue 3, 2016
For Rapid Diagnosis of Heart Attack at the Point of Care
Chest pain patients presenting at the emergency department are set to benefit from a major development by Royal Philips. The company has CE marked its cardiac troponin I (cTnI) blood test on the Philips Minicare I-20 handheld device. Current guidelines for the diagnosis of myocardial infarction (MI) require blood test results of cardiac troponin for the 90% of patients who present at the ED with chest pain, but are not diagnosed by an electrocardiogram (ECG). For high-risk cardiac patients (such as those with Acute Coronary Syndrome), fast triage and rapid initiation of treatment are critical in order to improve patient outcomes and to save lives.
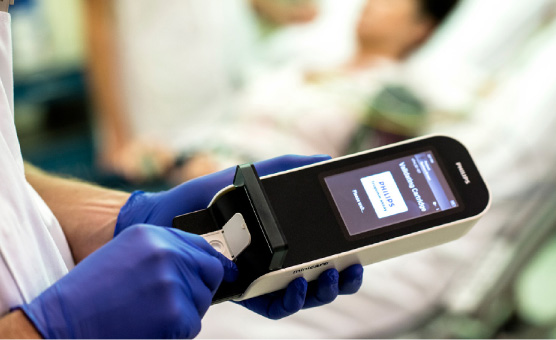
Minicare cTnI delivers lab comparable test results in less than 10 minutes, from only a single droplet of blood. The result is delivered near the patient, while they are being assessed and a medical history taken, reducing the time the physician needs to decide on treatment. This simplifies the patient-doctor interaction, helping physicians provide the best possible care for their patients.
Only 10% of chest pain patients can be diagnosed by ECG. The rest need to rely on additional cardiac marker testing for the diagnosis of MI. Physicians often have to wait up to six hours before deciding if they can safely discharge the patient or if they need to admit for further tests. The use of Minicare cTnI supports a reduction of the diagnostic protocol by up to three hours.
Lab2Go Study confirmation
The Minicare I-20 was tested in real life acute care settings within the European project Lab2Go, a consortium of European hospitals. The study showed the potential of the Philips Minicare cTnI to accurately measure cTnI values, near the patient in the emergency department, with a turn-around-time of less than 10 minutes.
Minicare I-20 is simple and easy to use by non-laboratory staff. The user is guided through the process steps by the intuitive interface and incorporated fail-safes. A droplet of blood from a finger prick or venous whole blood tube can be applied directly on the cartridge. Connectivity allows for direct transfer of the data to the laboratory or hospital information systems to update the patient files. The robustness and accuracy that is needed for confident decision making is provided by integrated calibration and fail-safe guarantees.
“Blood samples are usually analysed in the hospital laboratory, which can easily take more than an hour to get the result back to the ED physician. Point of care testing can significantly help to reduce the turnaround time,” said Dr. Paul Collinson, consultant chemical pathologist at St George’s University Hospitals NHS Foundation Trust.
“Minicare I-20 is designed to help care providers to reduce time to treatment and reduce time to discharge of patients, thereby helping to decrease crowding in the emergency department and leading to better use of hospital resources,” said Marcel van Kasteel, CEO of Handheld Diagnostics at Philips.
Performance
The clinical and analytical performance of Philips Minicare cTnI was validated during multicentre clinical studies. Minicare cTnI point of care assay showed excellent clinical performance for the diagnosis of myocardial infarction comparable to central laboratory assays. Minicare cTnI can be used as an aid in the diagnosis of an acute myocardial infarction (AMI) in a diagnostic protocol, including a cTn test, when the patient presents at the emergency department; and 2-4 hours later (0/3h diagnostic protocol) in appropriate patients. This follows the 2015 European Society of Cardiology (ES C) guidelines for the management of NSTE -ACS patients.
The Philips Minicare I-20 analyser and Minicare cTnI test
cartridges are sold in selected countries in Europe, including Austria,
Belgium, Denmark, Germany, Netherlands, Norway, Sweden, UK and Switzerland.
Please contact your Philips representative for availability in your country.
Philips Minicare I-20 at ESC 2016
Rome, August 27-31, 2016
Booth #E4-N100


