The role of the environment in the transmission of healthcare-associated infections (HCAIs) is increasingly recognised, requiring a new approach to the selection of materials for objects frequently touched by healthcare workers, patients and visitors that can serve as reservoirs of infection.
There are many technologies and materials on the market, but none are as effective under typical indoor conditions as copper, and its hygienic properties are far from new to us. The Ancient Egyptians, Greeks and Romans used copper-based preparations to treat ailments and prevent wound infections, and in India drinking water is traditionally stored in pots made of brass — an alloy of copper and zinc. Evidence shows that upgrading the most frequently-touched surfaces in a healthcare environment to antimicrobial copper can reduce the spread of costly infections and improve patient care. This article explores the growing body of research — from laboratory tests and clinical trials — and considers the practicalities and economics of upgrading key surfaces to copper.
Effective Under Typical Indoor Conditions
Copper’s antimicrobial properties have been documented in scientific literature for more than a century, but it was not until 2000 that its efficacy against the pathogens responsible for HCAIs began to be assessed.
Fifteen years on, more than 60 papers report copper’s broad-spectrum, rapid efficacy against bacteria, viruses and fungi — including MRSA, E. coli, Influenza and norovirus. No pathogen tested has been able to survive on copper.
Claims of antimicrobial efficacy made for many antimicrobial products are based on Japanese Industrial Standard (JIS) Z 2801, Antibacterial products - Test for antibacterial activity and efficacy (Japanese Standards Association 2012) and International Organization for Standardization (ISO) 22196: 2011 - Measurement of antibacterial activity on plastics and other non-porous surfaces (ISO 2011) tests, conducted at >90 percent humidity, 35oC and over 24 hours under a plastic film. These basic tests are described as a proof of principle and do not indicate how a material will perform in the field.
To better represent actual in-use conditions when testing copper, researchers developed new protocols to reflect typical room temperature and humidity and used representative contaminants.
The U.S. Environmental Protection Agency (EPA) approved one such test method and developed further protocols — including a challenging recontamination test — leading to the registration of hundreds of copper alloys to be marketed in the U.S. with public health claims. These were the first solid materials to achieve such recognition. As a general rule, alloys should have a minimum 60 percent copper content, and the higher the copper content, the faster the kill (in laboratory tests).
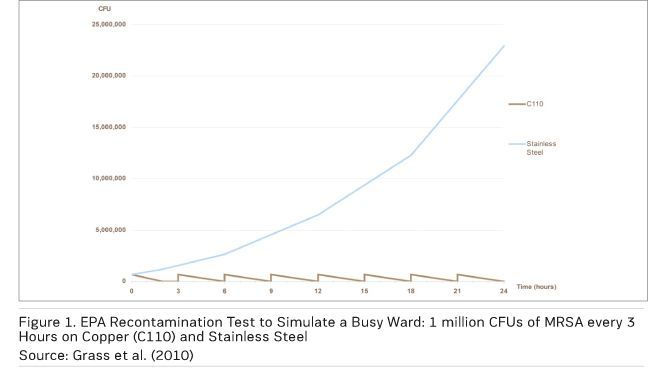
Figure 1 shows the results of an EPA recontamination test simulating a splash or sneeze — a ‘wet’ contamination event — with MRSA applied every three hours over a 24-hour period at room temperature and humidity. The number of MRSA used (1 million colony forming units per square inch) is far higher than would be found in a typical contamination event. On copper, the MRSA are totally eliminated before the next recontamination, while there is survival and significant growth on the stainless steel control (Michels et al. 2008).
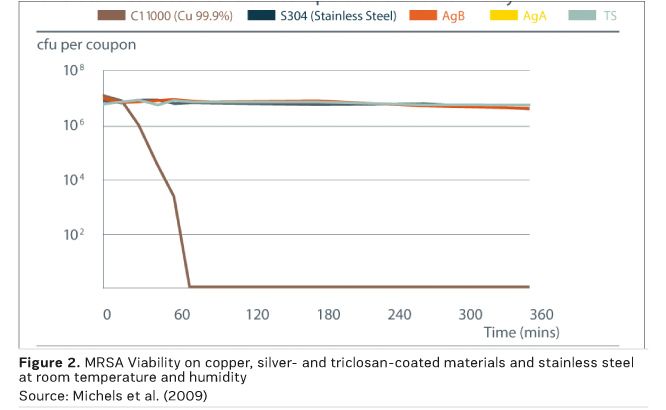
Laboratory research on the antimicrobial efficacy of copper and copper alloys has been carried out and verified at institutions around the world, with results peer-reviewed and published in respected journals. They exhibit efficacy under typical indoor conditions, unlike silver-containing materials and triclosan, which showed no antimicrobial efficacy under these conditions, as shown in Figure 2 (Michels et al. 2009).
Simulations of ‘dry’ touch contamination events have also been developed, and these tests show an even more rapid kill, with 106 vancomycin-resistant enterococci (VRE) killed in less than 10 minutes on 1cm2 copper (Warnes et al. 2011).
A leading researcher in this field is
Professor Bill Keevil, Chair in Environmental Healthcare at the University of Southampton, and his work includes investigation of the mechanisms by which copper exerts its antimicrobial effect.
For bacteria, the current consensus among researchers is that there are several probably interacting mechanisms, including:
- Causing leakage of potassium or glutamate through the outer membrane of bacteria;
- Disturbing osmotic balance;
- Binding to proteins that do not require copper;
- Causing oxidative stress by generating hydrogen peroxide;
- Degradation of bacterial DNA.
There is also agreement that bacteria will not develop resistaance to copper. Professor Keevil explains: “Copper works in completely different ways to antibiotics or common biocides. It punches a hole in the cell membrane, like a balloon, and the bacteria collapse. It stops them respiring, goes into the cell and destroys their DNA. Mutation happens because you get small changes in DNA in cells. The beauty of copper is it destroys the DNA; there is nothing left. We’ve shown this for bacteria, fungi and viruses. They can't mutate. They have no time.”
Most recently, the Southampton team has investigated the contribution antimicrobial copper surfaces can make to combating the rise of antibiotic resistance, assessing the ability of two different strains of bacteria to pass genetic material conveying antibiotic resistance between them on copper and stainless steel. While this took place on stainless steel, it did not happen on copper (Warnes et al. 2012). Copper can therefore contribute to the fight against antibiotic resistance in two ways: by reducing the spread of infections and thus the need for antibiotics and by preventing the transfer of resistance between bacteria on surfaces.
Proven Under Challenging Clinical Conditions
Having established the inherent ability of copper to eliminate bacteria and viruses in the laboratory, the next logical step was to discover how this would translate into real clinical environments. It is important to note that trials have used solid materials, as the effective surface will not wear away or be susceptible to reduced efficacy over time, as with coatings and composites.
Pathogens persist on standard clinical touch surfaces, creating reservoirs of infection that pose a risk to patients, staff and visitors, for days, weeks or even months. The first clinical trial – undertaken at Selly Oak Hospital in Birmingham, UK – found that antimicrobial copper taps, toilet seats and door handles on a general medical ward had 90 to 100 percent fewer bacteria on them than the same items made from standard materials (Casey et al. 2009).
Numerous trials have since been conducted in different healthcare systems — including the U.S., germany and Finland — and different clinical environments such as nephrology, geriatric and ICU wards. They have similarly reported significant and continuous bioburden reduction, with trial leaders concluding that antimicrobial copper surfaces can provide an additional measure to reduce the spread of HCAIs.
A multicentre clinical trial in ICUs, funded by the U.S. Department of Defense, took the research one step further and asked the question "Will the bioburden reduction associated with the installation of copper surfaces reduce the number of infections?" Led by
Dr. Michael Schmidt, Professor and Vice Chair of Microbiology and Immunology at the Medical University of South Carolina, the trial team found that replacing just six key, near-patient touch surfaces reduced the incidence of infections by 58 percent (Salgado et al. 2013). Figure 3 shows the accompanying reduction in microbial burden on the six surfaces (Schmidt et al. 2012).
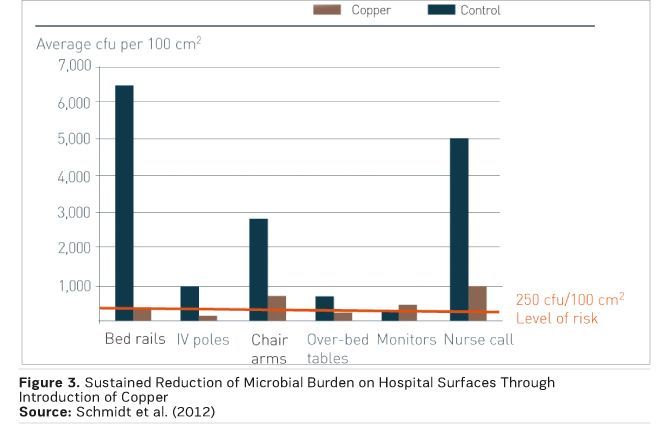
Just 10 percent of touch surfaces were upgraded to antimicrobial copper, yet the impact was significant. This study is the first to report a correlation between environmental bioburden (whether in copper or control rooms) and the risk of acquiring an infection, and to show a reduction in that risk due to a minimal intervention with an effective antimicrobial material.
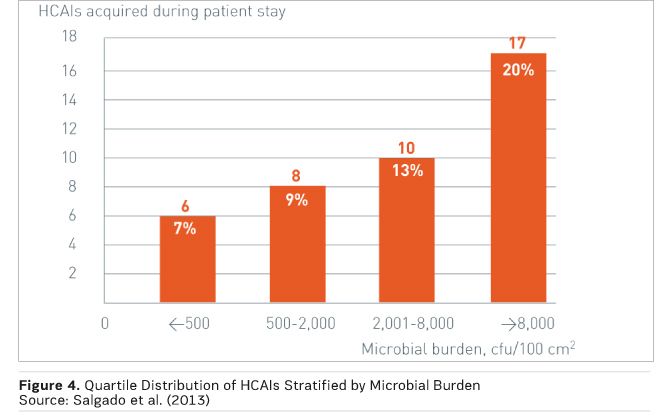
Figure 4 demonstrates this correlation, with quartile distribution of HCAIs stratified by microbial burden measured in the ICU room during the patient’s stay. There was a significant burden association between burden and HCAI risk, with 89 percent of HCAIs occurring among patients in rooms with a burden of more than 500 colony-forming units (CFU) per 100 cm2 (Salgado et al. 2013).
Key healthcare watchdogs and horizon scanning bodies around the world, including ECRI Institute (2014) and the Canadian Network for Environmental Scanning in Health (Ndegwa 2015) have recognised the growing body of evidence for copper’s potential to boost infection control. It has also been acknowledged in the evidence-based epic3 guidelines, which included copper as an emerging technology in 2014 (Loveday et al. 2014).
With this proven efficacy in mind, the next question arising will naturally concern the cost of installing antimicrobial copper touch surfaces.
Cost Benefits of Upgrading to Copper
HCAIs are very common and very costly, both financially and in terms of human life. Approximately 20 percent of ICU patients in European hospitals get HCAIS, and in 2011 they affected 4.1 million patients, necessitating 16 million extra days in hospital. Thirty-seven thousand deaths were recorded as being caused by HCAIs, plus 110,000 deaths where they were a contributing factor, and they had a direct clinical cost in excess of 7 billion euros (World Health Organization 2011).
York Health Economics Consortium (YHEC), a group of leading global health economists based at the University of York in the UK, developed a fully-referenced cost benefit model for hospital managers to illustrate the economic rationale of an antimicrobial copper installation (Taylor et al. 2013). The model is based on the cost of installing antimicrobial copper touch surfaces and the balancing cost savings resulting from reduced infection rates. The model allows local data to be entered for site-specific evaluations, but is populated with default data for the UK as an illustration.
Using UK data for cost of infection, industry data for cost of antimicrobial copper and standard components, and a conservative infection rate reduction of 20 percent (where the U.S. trial reported a 58 percent reduction), the model considers a planned refurbishment or new build. It predicts that the cost of replacing the six key touch surfaces in a 20-bed ICU with antimicrobial copper equivalents will be recouped in less than two months, based on fewer infections and the resulting shorter lengths of stay. It also calculates a positive impact on bed days and quality-adjusted life years offered by antimicrobial copper.
Dr. Matthew Taylor, YHEC’s director and one of the model’s authors, concludes: “After the initial two months, ongoing cost savings will accrue from the reduction in blocked beds and better directed staff resources.”
Specifying Copper
There is an ever-expanding range of products on the market as the supply chain responds to growing demand, so how does one get started with selecting the priority touch surfaces to upgrade in a given healthcare environment?
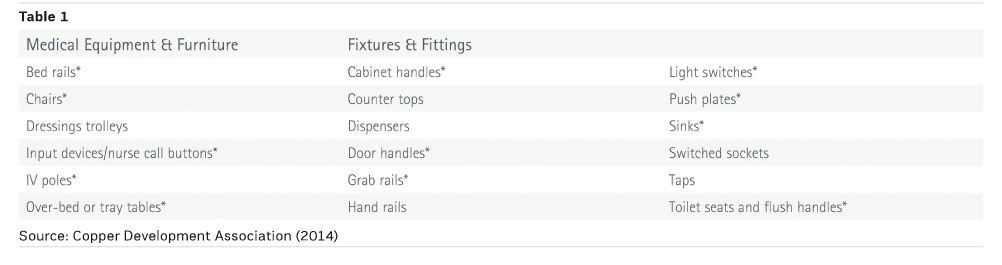
A number of studies have identified frequently-touched surfaces as being contamination hotspots that present an infection risk and are therefore targets for upgrade. Based on a review of international research, the United States Centers for Disease Control and Prevention (CDC) published a checklist of key surfaces based upon the likelihood of touch and contamination (guh et al. 2015).
In the many copper clinical trials, conducted around the world, multidisciplinary teams have prioritised high frequency touch surfaces to upgrade to copper. The factors considered include known hotspots from microbiological testing and likely hotspots based on experience and understanding of staff/ patient/visitor dynamics.
Table 1 represents a summary of these surfaces with CDC surfaces indicated by an asterisk, to differentiate from those identified in clinical trials, and is the starting point for selecting items to upgrade for any new build or refurbishment project.
Input should also be sought from the infection control team and ward staff to ensure that all high-risk touch surfaces specific to a particular area are included. The regular environmental swabbing carried out by infection control teams to assess the state of cleanliness will also indicate contamination.
Support with identifying efficacious products is available in the form of an industry stewardship scheme. The Antimicrobial Copper brand and Cu+ mark are used by leading manufacturers of hospital equipment, furniture and fittings to indicate their products are made from solid antimicrobial copper, and that the organisation adheres to strict usage rules guiding their understanding of the underlying technology and its deployment.
Copper alloys offer a wide palette of colours from the gold of brasses to the rich brown of bronzes right through to the silver/white shades of copper-nickels. Copper alloys will naturally darken over time, but this does not impact their antimicrobial efficacy. More colour-stable alloys traditionally used in naval applications are available.
Wide Installation
Antimicrobial copper surfaces are an adjunct to and not a replacement for existing infection control measures. Alongside good hand hygiene and regular surface cleaning and disinfection, they will continuously reduce surface contamination and consequently the risk of infections being passed between people via these surfaces.
Installations have already taken place around the world in more than 25 countries. In these hospitals, the importance of taking a multidisciplinary approach to infection control has been clear.
Casey AL, Adams D, Karpanen TJ et al. (2009) Role of copper
in reducing hospital environment contamination. J Hosp Infect, 74(1): 72-7.
Copper Development Association (2014) Reducing the risk of
healthcare associated infections: the role of antimicrobial copper touch
surfaces. Hemel Hempstead: Copper Development Association. [Accessed: 25
October 2015] Available from antimicrobialcopper.org/media/69544/pub196reducing-risk-healthcare-infections.pdf
ECRI Institute (2014) Top 10 hospital C-suite watch list
2014. Plymouth Meeting, PA: ECRI Institute.
Grass G, Rensing C, Siolz M (2010) Metallic copper as an
antimicrobial surface. Appl Environ Microbiol, 77(5): 1541-7.
Guh A, Carling P, Environmental Evaluation Workgroup (2010)
Options for evaluating environmental cleaning. In: Preventing HAIs: toolkits. Atlanta,
GA: Centers for Disease Control and Prevention. [Accessed: 25 October 2015]
Available from: cdc.gov/HAI/toolkits/EvaluatingEnvironmental-Cleaning.html
International Organization for Standardization (2011) ISO 22196: 2011 - Measurement of
antibacterial activity on plastics and other non-porous surfaces (ISO 2011)
Japanese Standards Association (2012) JIS Z 2801: 2012.
Antibacterial products-Test for antibacterial activity and efficacy. Tokyo:
Japanese Standards Association.
Loveday HP, Wilson JA, Pratt RJ et al. (2014) epic3:
national evidence-based guidelines for preventing healthcare-associated
infections in NHS hospitals in England. J Hosp Infect, 86 Suppl 1: S1-70.
Michels HT, Anderson D (2008) Antimicrobial regulatory
efficacy testing of solid copper alloy surfaces in the USA. Metal Ions in
Biology and Medicine, 10: 18590. John Libbey Eurotext: Paris.
Michels HT, Joyce JO, CW Keevil (2009) Effects of
temperature and humidity on the efficacy of methicillin-resistant
Staphylococcus aureus challenged antimicrobial materials containing silver and
copper. Lett Appl Microbiol, 49(2): 191-5.
Ndegwa S Antimicrobial copper surfaces for the reduction of
health care–associated infections in intensive care settings. Issues in
emerging health technologies, 133.Ottawa: Canadian Agency for Drugs and
Technologies in Health; 2015. [Accessed: 25 October 2015] Available from
cadth.ca/sites/default/ files/pdf/EH0021_Copper_Surfaces_e.pdf
Salgado CD, Sepkowitz KA, John JF et al. (2013) Copper
surfaces reduce the rate of healthcare-acquired infections in the Intensive
Care Unit. Infect Control Hosp Epidemiol, 34(5): 479-86.
Schmidt MG, Attaway HH, Sharpe PA et al. (2012) Sustained
reduction of microbial burden on common hospital surfaces through introduction
of copper. J Clin Microbiol, 50(7): 2217-23.
Taylor M, Chaplin S (2013) The economic assessment of an
environmental intervention: discrete deployment of copper for infection control
in ICUs. Antimicrobial Resistance and infection control, 2 (Suppl 1): P368.
[Accessed: 15 September 2015] Available from
aricjournal.com/supplements/2/S1/all
Warnes SL, Keevil CW (2011) Mechanism of copper surface
toxicity in vancomycin-resistant enterococci following wet or dry surface
contact. Appl Environ Microbiol, 77(17): 6049–59.
Warnes SL, Callum JH, Keevil CW (2012) Horizontal transfer
of antibiotic resistance genes on abiotic touch surfaces: implications for
public health. [Accessed: 15 September 2015] Available from
mbio.asm.org/content/3/6/e00489-12.full.html