The aim of administering contrast media in medical imaging is to improve image contrast and the diagnostic efficacy of the imaging examination being undertaken. Developments in the manufacture of iodinated contrast media have led to an increase in safety standards. However, the administration of iodinated contrast media still provides a potential small risk to the patient.
The purpose of this review is to provide awareness about:
- Adverse side effects from the administration of iodinated contrast media;
- Preparation and planning for reducing and treating adverse effects of iodinated contrast media;
- Quality assurance and quality improvement programmes for the administration of iodinated contrast media.
The use of iodinated contrast media during imaging examinations has increased considerably in recent years. Administration of iodinated contrast media carries the risk of contrast-induced acute kidney injury (CI-AKI) and nonrenal adverse reactions. The minimal possible risks associated with the use of iodinated contrast media should be assessed against the benefits of their use in providing the required diagnosis and eventual appropriate patient management (Royal College of Radiologists (RCR) 2015; American College of Radiologists (ACR) 2013; Royal Australian and New Zealand College of Radiologists (RANZCR) 2009). Alternatives, which could provide the same or better diagnosis and justification based on accurate clinical indications, should be considered (RANZCR 2009).
Guidelines on the safe use of iodinated contrast media have been published with the aim of reducing the risks during their administration (RCR 2015; ACR 2013; RANZCR 2009).
Documented incidence of adverse reactions due to the administration of iodinated intravenous contrast is low (ACR 2013), and therefore the need for informed consent varies in different countries. Policy on informed consent should be based on the legal requirements of the country and institutional and departmental policies (ACR 2013).
Adverse Effects of Iodinated Contrast Media and Their Management
Adverse effects from the administration of iodinated intravenous contrast media vary from mild reactions to rare but severe life-threatening situations.
Contrast-induced acute kidney injury (Ci-AKI)
Contrast-induced acute kidney injury (CI-AKI) has replaced other terminology such as contrast nephrotoxicity, contrastinduced nephropathy (CIN) or radiocontrast nephropathy (RCN). CI-AKI is the third leading cause of hospital-acquired acute renal failure (Kagan 2010). It is an iatrogenic disease, which may cause long term morbidity or death. CI-AKI is defined when one of the following criteria is met:
- serum creatinine rises by ≥26 µmol/l within 48 hours after administration of contrast medium compared to baseline creatinine values;
- serum creatinine rises ≥1.5 fold from the baseline value, which is known or presumed to have occurred within one week after administration of iodinated contrast medium;
- urine output is <0.5 ml/kg/hour for more than 6 consecutive hours;
- when alternative explanations for renal impairment have been excluded.
These variable definitions make it difficult to predict the long-term clinical outcome of CI-AKI. CI-AKI is both an adverse effect that may permanently impair renal function, increase the length of hospital stay and hospital costs as well as a predictor of future adverse cardiovascular events and mortality (RCR 2015; Richenberg 2012).
Acute reactions
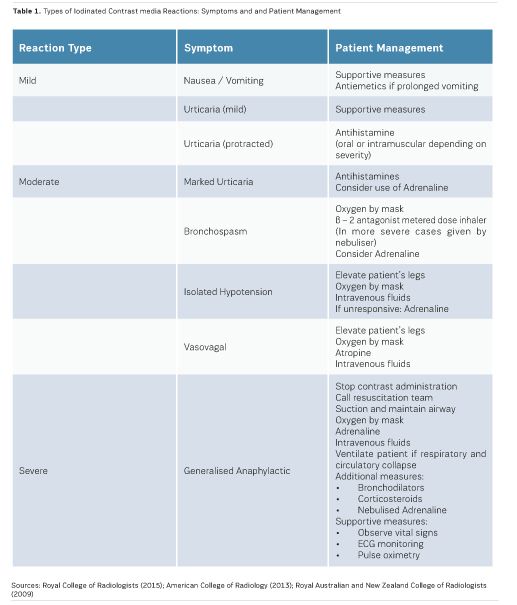
Acute reactions are those which take place within 60 minutes of the administration of iodinated contrast media. The majority of acute reactions are anaphylactoid, are not dose-dependent, and involve the release of histamine along with other active biological chemicals. Acute reactions may be classified as mild, moderate and severe (RCR 2015):
- Mild reactionsmay be experienced by up to three percent of patients following iodinated contrast media administration. Such reactions usually resolve without the need for any treatment, and include nausea, pruritus, vomiting, marked urticaria, headache, and mild urticaria (RCR 2015; ACR 2013; RANZCR 2009; Bettmann 2004).
- Moderate reactions may require specific treatment, and include severe vomiting, marked urticaria, bronchospasm, facial/laryngeal oedema and vasovagal attacks (RCR 2015; ACR 2013; RANZCR 2009; Bettmann 2004).
- Severe reactionsoccur in 0.04% to 0.004% of iodinate contrast media administrations, with the risk of death being rare. Severe reactions include hypovolaemic shock, respiratory arrest, cardiac arrest, pulmonary oedema and convulsions (RCR 2015; ACR 2013; RANZCR 2009; Bettmann 2004).
Delayed reactions
Delayed reactions, which account for less than four percent of reactions, take place between one hour to one week after the administration of iodinated contrast media. Delayed reactions most commonly present as skin reactions with a maculopapular rash. Less frequent skin reactions include angioedema, urticaria and erythema (RCR 2015; ACR 2013; RANZCR 2009; Bettmann 2004).Table 1 presents the types of iodinated contrast media reactions and suggested patient management.
Minimising the Risks of Iodinated Contrast Media Administration
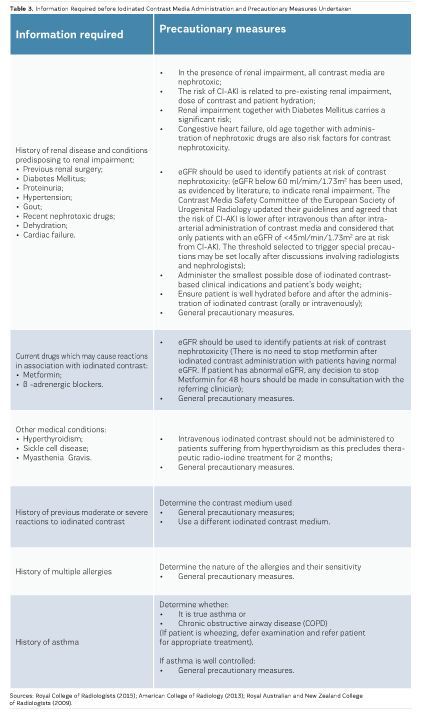
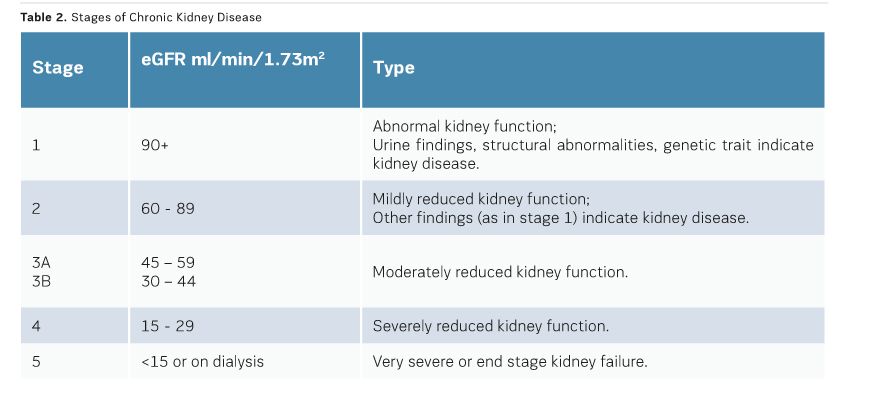
True CI-AKI is defined when there is a permanent reduction in renal function as opposed to a temporary alteration of renal function post iodinated contrast medium administration. True CI-AKI is rare and usually confined to individuals with pre-existing renal dysfunction. The risk of CI-AKI is 0.6% to 2.6% in the general population, but rises to 4.7% in patients with pre-existing renal impairment. Renal impairment, measured best by estimated glomerular filtration rate (egFR), is the only risk factor predictive of CI-AKI. Patients who have a high risk for CI-AKI need to be identified prior to iodinated contrast media administration through measurements of egFR. Renal impairment is considered in patients with an egFR <60ml/mim/1.73m2. However, the Contrast Media Safety Committee of the European Society of Urogenital Radiology, in their updated guidelines, agreed that the risk of CI-AKI is lower after intravenous than after intra-arterial administration of contrast media. They considered that only patients with an egFR of <45ml/min/1.73m2 are at risk from CI-AKI after intravenous administration. Other risk factors, such as age over 75 years, diabetes, metformin use, congestive heart failure, gout and collagen vascular disease should also be considered (Richenberg 2012; Stacul 2011).
To reduce the risk of CI-AKI, egFR measurements should be available for all nonemergency cases. An egFR within the previous three months is satisfactory for patients in a stable condition. Patients with known renal disease should have an egFR measurement from the previous seven days, which can be compared against a baseline. In the presence of chronic kidney disease (egFR<40ml/ min/1.73m2), one should consider temporarily stopping angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers. Consultation with a nephrologist has been shown to be beneficial for patients at risk of CI-AKI (RCR 2015; Lewington, 2013). (See Table 2 for stages of chronic kidney disease).
To date, the best known preventive measure to reduce the risk of CI-AKI is hydration. The smallest dose of low osmolar non-ionic monomeric or isoosmolar non-ionic dimeric contrast medium should be used. There is insufficient evidence to suggest routine use of any other pharmacological agent in attempting to reduce CI-AKI (RCR 2015).
Mild reactions to iodinated contrast are relatively common, but are mostly selflimited and of no consequence. Severe reactions, although rare, can occur in the absence of any specific risk factor with any type of contrast media (RCR 2015; ACR 2013; RANZCR 2009). Therefore the aims during the administration of iodinated contrast are to:
- Ensure that the administration of iodinated contrast is appropriate for the patient and the clinical indication;
- Reduce the possibility of a reaction;
- Be fully prepared to promptly recognise and treat a reaction if this occurs.
General safety considerations during the administration of iodinated contrast include the availability of an appropriately trained doctor, who can promptly deal with a severe reaction. Those administering iodinated contrast media should be able to identify the symptoms of severe reactions. Patients should be well hydrated prior to iodinated contrast media administration. Facilities for the treatment of acute reactions should be readily available and regularly checked. No patient should be left unsupervised within the first five minutes post administration of iodinated contrast. It is good practice for patients to remain on the premises for at least 15 minutes post administration of iodinated contrast as, based on evidence, most severe reactions occur during this period (RCR 2015). This period should be 30 minutes for individuals who are at an increased risk of adverse reactions. All types of reactions should be reported and documented for future reference (RCR 2015; ACR 2013; Richenberg, 2012; Owen et al. 2014; RANZCR 2009; Bettmann 2004).
Whilst there is always a potential risk for all individuals undertaking iodinated contrast media examinations, there should be a system whereby individuals who pose an increased risk can be identified so that adequate planning and precautionary measures can be taken before their administration. In the presence of an increased risk, the decision about iodinated contrast media administration should be taken by the supervising radiologist based on adequate information (Royal College of Radiologists 2015; American College of Radiology 2013; Owen et al., 2014; RANZCR 2009). Information aiding the identification of individuals who pose an increased risk from the administration of iodinated contrast together with precautionary measures for each case is presented in Table 3. For all such cases, if administration of iodinated contrast is still considered necessary, the following general precautionary measures should be taken:
- Supervise patient continuously;
- Leave cannula in place for at least 30 minutes post administration;
- Ensure availability of emergency drugs and equipment.
Specific precautionary measures are also included in Table 3.
If iodinated contrast administration is necessary during pregnancy, there is a small risk of thyroid suppression to the fetus, and therefore a thyroid function test should be performed during the first week after birth (Royal College of Radiologists 2015).
A small percentage of iodinated contrast is passed on to breast milk in lactating mothers. However, no specific precaution is necessary and mothers may continue to breastfeed their infants without any significant risk (Royal College of Radiologists 2015).
Preparation for the treatment of iodinated contrast media reactions must include preparation for the whole variety of possible reactions and include availability of appropriately trained personnel, medications and equipment (RANZCR 2009)
Quality Assurance and Quality Improvement Programmes
In terms of the administration of iodinated contrast media, quality assurance relates to the systematic monitoring and evaluation of the various aspects of administration, to ensure that required standards of quality are being met and continuously maintained. To ensure patient safety during the administration of iodinated contrast, resuscitation equipment, and medications for the treatment of complications should be made readily available and regularly checked and maintained (RANZCR 2009).
All personnel who perform venepuncture for the administration of iodinated contrast should be well trained in: (Royal College of Radiologists 2015)
- Venepuncture procedures and have received formal certification of their competence;
- The recognition of iodinated contrast reactions and the procedures for their treatment;
- Cardiopulmonary resuscitation (CPR).
The competencies of personnel should be updated and maintained through training programmes as part of their continuous professional development (CPD).
Conclusion
Recognition of the early signs of iodinated contrast media adverse effects, the risk of reactions to pre-existing conditions, taking adequate precautions to minimise risks and provision of prompt and adequate treatment ensures optimal patient safety and care.
Key Points- Iodinated contrast media adverse effects and risk recognition;
- Precautions to minimise risks;
- Preparation and planning for reducing and treating adverse effects.
See Also: Contrast Media and Risk Management: Develop Simple Rules of Conduct
American College of Radiology (2013) ACR manual on contrast
media. Version 9. Reston, VA: American College of Radiology.
Bettmann MA (2004) Frequently asked questions: iodinated
contrast agents. RadioGraphics., 24(Suppl 1): S3-10.
Kagan A, Sheikh-Hamad D (2010) Contrast-induced kidney
injury: focus on modifiable risk factors and prophylactic strategies. Clin
Cardiol, 33(2): 62-6.
Lewington A, MacTier (2013) Prevention of contrast induced
acute kidney injury in adult patients. Renal Association; British
Cardiovascular Intervention Society; Royal College of Radiologists. [Accessed:
12 October 2015] Available from rcr.ac.uk/ publication/prevention-contrast-inducedacute-kidney-injury-ci-aki-adult-patients
Owen RJ, Hiremath S, Myers A et al. (2014) Canadian
Association of Radiologists consensus guidelines for the prevention of contrast
induced nephropathy: update 2012. Can Assoc Radiol J, 65(2): 96-105.
Royal Australian and New Zealand College of Radiologists
(2009) RANZCR guidelines for iodinated contrast administration. Sydney: Royal
Australian and New Zealand College of Radiologists. [Accessed: 12 October 2015]
Available from ranzcr.edu.au/component/ docman/?task=doc_download&gid=573
Richenberg, J. (2012) How to reduce nephropathy following
contrast-enhanced CT: a lesson in policy implementation. Clin Radiol, 67:
1136-45.
Royal College of Radiologists (2015) Standards for
intravascular contrast agent administration to adult patients. 3rd ed. London:
Royal College of Radiologists. [Accessed: 12 October 2015] Available from
rcr.ac.uk/publication/standards-intravascular-contrastadministration-adult-patients-third-edition
Stacul F, van der Molen AJ, Reimer P et al. (2011) Contrast
induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines.
Eur Radiol, 21, 2527-41.