HealthManagement, Volume 14 - Issue 2, 2014
Authors:

Dr. Demosthenes D. Cokkinos

Dr. Eleni G. Antypa

Dr. Ploutarchos N. Piperopoulos
Ultrasound (US) contrast agents are used extensively worldwide. In order to moderate their application, a first set of guidelines for contrast enhanced ultrasound (CEUS) was published in 2004 by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB), focusing on liver imaging (Albrecht et al. 2004). In 2008, new EFSUMB guidelines (Claudon et al. 2008) reflected changes in available agents, updated liver indications and introduced extrahepatic applications. In 2012 a joint World Federation for Ultrasound in Medicine and Biology (WFUMB)-EFSUMB initiative resulted in updated liver guidelines (Claudon et al. 2013). A year earlier, another set of EFSUMB guidelines studied CEUS for non-liver imaging (Piscaglia et al. 2012). This article assesses the role of these guidelines in common CEUS indications, with the addition of other international guidelines when applicable.
1. Liver
1.1. Focal Liver Lesions (FLLs)Characterisation
CEUS is performed using three
different agents:
1. SonoVue (sulfur hexafluoride
with a phospholipid shell).
2. Definity/Luminity
(octafluoropropane [perflutren] with a lipid shell).
3. Sonazoid (perfluorobutane with
a phospholipid shell: hydrogenated egg phosphatidyl serine).
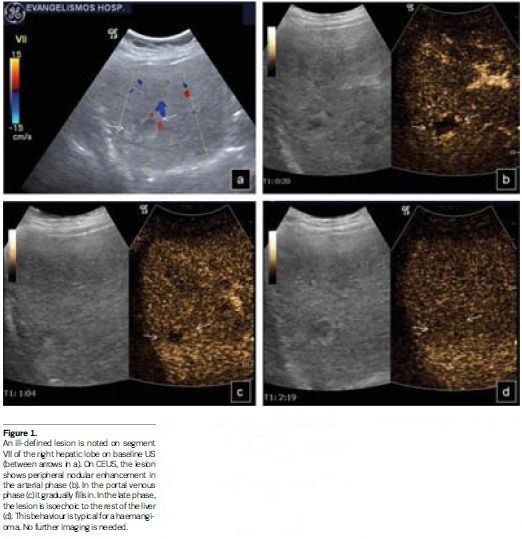
CEUS has high specificity for characterising FLLs (von Herbay et al. 2004; Bleuzen and Tranquart 2004; Ding et al. 2005; Leen et al. 2006), studying enhancement in the arterial, portal and late parenchymal phases. Sonazoid liver enhancement is longer, following a postvascular ‘Kupffer phase’ (Yanagisawa et al. 2007). CEUS is the imaging examination that should be initially performed when a FLL is located on baseline US (Beaton et al. 2010), since it shows the highest sensitivity, specificity and accuracy (Trillaud et al. 2009). When enhancement patterns are typical for specific FLLs (haemangioma, focal nodular hyperplasia, focal fatty change/sparing, certain malignancies), these can be characterised (Claudon et al. 2013) with no additional imaging (see Figure 1). When atypical patterns are noted on CEUS, further examination is warranted.
For hepatocellular carcinoma (HCC) study, CEUS has a controversial role. The American Association for the Study of Liver Diseases (AASLD) guidelines (Bruix et al. 2011) suggest an US-based HCC surveillance protocol. In cirrhosis arterial hyperenhancement and late washout on CEUS is crucial for HCC characterisation (Bruix et al. 2011). This pattern corresponds to 97% of HCCs (Boozari et al. 2011; Fan et al. 2006; Foschi et al. 2010; Vilana et al. 2010), but is also seen in peripheral cholangiocarcinoma (CCC) and hepatic lymphoma. Late washout is observed more frequently in poorly differentiated HCCs. Well differentiated HCCs are more isoechoic in the late phase (Boozari et al. 2011; Fan et al. 2006; Iavarone et al. 2010; Jang et al. 2007). CEUS HCC washout is observed less often than CT/MR, due to different contrast pharmacokinetics (Forner et al. 2008). Inconclusive CEUS does not exclude malignancy and should proceed to CT/MR. If these are also inconclusive, biopsy is performed. CEUS, as first-line investigation, is part of the Japanese HCC guidelines, (Kudo et al. 2001b; Kudo and Okanoue 2007), but has been removed from the AASLD guidelines (Bruix et al. 2011). This is partly justified by the still non-existent licence for liver CEUS in the USA and difficulty in differentiating CCC from HCC by CEUS alone, since both lesions show comparable dynamic perfusion patterns (Piscaglia et al. 2006). This risk is minimal when CEUS is performed by skilled operators (Barreiros et al. 2012). CEUS is not included in the European Association for the Study of the Liver (EASL)-European Organisation for Research and Treatment of Cancer (EORTC) Clinical Practice Guidelines for HCC (European Association for the Study of the Liver and European Organisation for Research and Treatment of Cancer 2012), and CT/MR are recommended for noninvasive HCC diagnosis (Piscaglia et al. 2007).
Guidelines of CEUS for characterisation of FLLs in the non-cirrhotic liver include:
• Characterising routine US
incidental findings;
• Lesions detected on US in patients with known malignancies, as a CT/MR alternative;
• Contrast enhanced C T/M Rcontraindication;
• Inconclusive CT/MR or cytology/ histology;
Guidelines of CEUS for FLL characterisation in the cirrhotic liver include:
• Characterisation of nodules
found on baseline US;
• Characterisation of nodules in cirrhosis. CT/MR are needed for pre-treatment staging;
• Variable international acceptance as a first-line investigation for HCC at the same level as CT/MR;
• Upon inconclusive CT or MR, especially in nodules unsuitable for biopsy;.
• Contribution to selection of nodule(s) for biopsy when these are multiple or show different contrast patterns;
• Monitoring changes in size and enhancement patterns over time when a nodule is not diagnostic for HCC;
• After inconclusive histology.
For Sonazoid, taken up by the reticuloendothelial
(Kupfer) cells, like superparamagnetic iron oxide MR contrast agents (Korenaga
et al. 2009), with a late phase beginning 10 minutes post injection, guidelines
include:
• Nodule characterisation in
cirrhosis, assessing vascular and post vascular phases. This practice is
adopted by Japanese guidelines for HCC management (Kudo et al. 2011; Kudo and Okanoue
2007; Kudo 2010);
• HCC screening in cirrhosis,
although not proven so far as cost-effective;
• HCC staging when US imaging is
satisfactory. No evidence exists so far that CEUS can replace contrast-enhanced
computed tomography (CECT)/ contrast-enhanced MR (CEMR).
1.2. Liver Metastasis Detection
CEUS improves metastasis detection compared to baseline US (Piscaglia et al. 2007; Cantisani et al. 2010; Dietrich et al. 2006; Larsen et al. 2007; Quaia et al. 2006), showing portal venous and late phase washout. With SonoVue and Definity/Luminity the examination lasts for at least 3-4 minutes. With Sonazoid, very late washout can be detected (Moriyasu and Itoh 2009). When a late phase enhancing defect is noted, a second injection shows arterial enhancement, confirming its metastatic nature.
Guidelines of CEUS for detection of malignant liver lesions include:
• Characterisation of
indeterminate lesions on CECT-CEMR;
• Ruling out of metastases or
abscesses;
• Treatment planning for assessing
metastases’ number and location;
• Oncology patients’ surveillance;
• Replacement of unenhanced US for
evaluating colorectal cancer patients post chemotherapy for metastases presence.
1.3. Portal Vein Thrombosis Characterisation
Portal vein thrombus may be bland (benign
intraluminal clot) or malignant, usually due to HCC (Piscaglia et al. 2010). On
CEUS, bland thrombus shows no uptake (Piscaglia et al. 2010; Ueno et al. 2006;
Rossi et al. 2006; Sorrentino et al. 2009). Malignant thrombus enhances parallel
to the originating tumour (quick arterial hyperenhancement-fast washout
(Piscaglia et al. 2010).
1.4. Liver Biopsy Planning
CEUS increases biopsy diagnostic
yield and decreases the false negative rate, especially in large tumours with
necrotic areas, differentiating high vascularity regions, which should be
targeted, from areas of necrosis, which should be avoided (Wu et al. 2006).
1.5. Intraoperative CEUS
Intraoperative ultrasound (IOUS) is
the gold standard for liver resection or transplantation (Conlon et al. 2003). Intraoperative
CEUS (IOCEUS) shows higher sensitivity, specificity and accuracy compared to
IOUS, CT or MR for defining if metastasis/HCC resection is needed, altering
surgical management in up to 30% of cases (Leen et al. 2006; Lu et al. 2008;
Torzilli et al. 2007).
Guidelines for IOCEUS include:
• Metastasis detection in patients
undergoing liver resection;
• Nodule characterisation in
cirrhotic patients undergoing resection for HCC, especially if IOUS reveals new
lesions;
•
Targeting occult lesions for ablation.
1.6. Monitoring Liver Ablation
CEUS aids in selecting lesions to
be ablated (Minami et al. 2007), depicting previously undetectable lesions and
identifying still viable tumours post-therapy.
Guidelines of CEUS for monitoring liver ablation include:
• Complementing CECT/CEMR for
pretreatment staging and lesion vascularity assessment;
• Immediate ablation result
estimation. Guidance for immediate re-treatment if residual unablated tumour is
detected;
• Local tumour progression
assessment for follow-up.
1.7. Liver Transplantation
Common transplantation complications are hepatic artery (HA) stenosis and thrombosis (Shaw et al. 2003). Portal vein (PV), hepatic vein (HV) and inferior vena cava (IVC) thrombosis are less common. CEUS is more sensitive than Doppler US in detecting these complications.
Guidelines of CEUS for liver transplantation include:
• Pre-transplantation assessment
of PV patency and FLL characterisation;
• Post-transplantation
confirmation of intrahepatic HA, PV, HV, IVC occlusion;
• If haematoma is present,
searching for ongoing haemorrhage;
• Infarction exclusion;
• Monitoring success of
thrombolysis post intervention for HA occlusion.
1.8. Contrast Quantification and Monitoring Hepatic Malignancy Treatment
Neoangiogenesis is an important feature
of tumour growth and a target for anticancer treatment. Dynamic CEUS (DCEUS)
monitors therapy response, relying on quantitative features, if standardisation
and strict scanner control settings are followed (Dietrich et al. 2012).
Quantification software (time-intensity curves), background subtraction and
specific calculations (time to peak intensity, mean transit time, peak
intensity, area under the curve etc,) (Peronneau et al. 2010) are mandatory for
this technique.
2. Spleen
Splenic CEUS is indicated for focal
lesion characterisation (Görg and Bert 2005). Incidental hyperechoic lesions in
asymptomatic people are usually benign. In cancer patients, multiple hypoechoic
lesions are often malignant.
Guidelines of CEUS in the spleen include:
• Parenchymal inhomogeneity or
suspected lesions on baseline US;
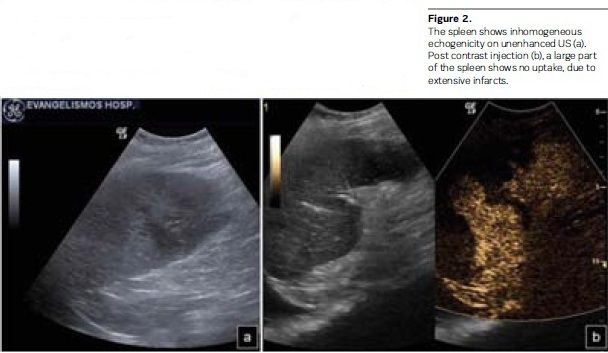
• Suspected infarction
confirmation (see Figure 2);
•Suspected accessory
spleen/splenosis characterisation;
• Oncologic patient malignancy
detection if CT/MR/PET are contraindicated or inconclusive.
3. Pancreas
CEUS is not helpful for focal lesion detection, but aids in their characterisation (Dietrich et al. 2008; D’Onofrio et al. 2007). Inflammatory lesions enhance along with, but malignancies are hypoechoic compared to adjacent pancreatic tissue. Pseudocysts show no enhancement, with CEUS sensitivity and specificity in their characterisation of 100% (Rickes and Wermke 2004).
Guidelines of CEUS in the pancreas
include:
• Ductal adenocarcinoma characterisation;
• Differentiation between
pseudocysts and cystic tumours;
• Differentiation between vascular
(solid) and avascular (liquid/necrotic) areas of a lesion;
• Defining of lesion dimensions
and margins;
• Distinction between solid and
cystic lesions;
•
Diagnosis when CT is indeterminate.
4. Kidneys
Renal infarction, traumatic injury
or haemorrhage appear as enhancing defects (Nilsson 2004). Excellent results
are observed studying atypical cysts with echogenic content, septa or nodules
and classifying them with the Bosniak system (Robbin 2001; Robbin et al. 2003),
with accuracy comparable to CECT (Quaia et al. 2008). Tumours enhance differently
than the rest of the kidney at least in one phase (Correas et al. 2006). Normal
variants, like a prominent Bertin septum (‘pseudotumours’), enhance parallel to
renal parenchyma. CEUS cannot differentiate between benign and malignant tumours
(Correas et al. 2006), but can characterise enhancing renal vein thrombosis as
neoplastic, as accurately as CECT (Ignee et al. 2010). Enhancing echogenic
material in the pelvicalyceal system is characterised as neoplastic, while pus
and blood do not enhance. Focal pyelonephritic oedematous areas show reduced
enhancement. Abscesses appear as non-enhancing lesions with peripheral uptake.
Guidelines of CEUS in the kidneys include:
• Suspected infarction and
cortical necrosis;
• Differentiation between solid
masses and cysts with atypical appearance on baseline US;
• Differentiation between tumours
and anatomical variations upon equivocal baseline US;
• Characterisation and follow-up
of complex cystic masses;
• Abscess detection;
• Lesion visualisation and
residual detection after tumour ablation.
5. Trauma
Blunt abdominal trauma is initially imaged with baseline unenhanced FAST (Focused Assessment with Sonography in Trauma) US (Kretschmer et al. 1997; Yoshii and Sato 1998; Healy et al. 1996), which is excellent for fluid detection, but has low sensitivity for revealing abdominal solid organ injuries (Pearl and Todd 1996). CECT (the best modality in this task) is often over-utilised and has its own disadvantages. Low energy localised trauma is well imaged with CEUS, revealing injuries undetected by baseline US (Valentino et al. 2009). Solid organ lacerations and haematomas appear as non enhancing areas (Afaq et al. 2012; Cokkinos et al. 2013). Although not replacing CT, CEUS is an alternative to reduce its use. Moreover, patients initially assessed with CT can be followed with CEUS with no additional CT performed (Cokkinos et al. 2013).
Guidelines of CEUS for blunt abdominal trauma include:
• Alternative to CT in stable
patients with isolated moderate energy solid abdominal organ trauma, especially
in children;
• Elucidation of uncertain CT
findings;
• Follow-up of trauma managed
conservatively, to minimise CT scanning.
6. Scrotum
CEUS is useful for ruling out
torsion and differentiating solid tumours from non enhancing testicular
contusions. Abscesses appear as non-enhancing lesions with peripheral uptake.
High frequencies (>7.5MHz) and high contrast doses (2.4-4.8 ml for SonoVue)
should be used.
Guidelines of CEUS in the scrotum include:
• Discriminating vascular from
nonvascular lesions;
• Imaging of non-viable tissue in
trauma;
• Detection and characterisation
of segmental testicular infarction;
• Detection of abscess in severe epididymoorchitis.
7. Paediatric Applications
In the past, the only agent
approved for paediatric use (only for vesico-ureteral reflux) was Levovist,
which is not produced anymore (Claudon et al. 2013). SonoVue, with equally good
results for this and all paediatric indications, is administered off-label,
since there is no current approval for children. This is a peculiar situation,
since one of the primary CEUS advantages is reduction of ionising radiation, of
grave importance for paediatric imaging. Strict regulations on paediatric CEUS
may inhibit the use of a very beneficial and simple technique (Piscaglia et al.
2012).
8. Other Applications
Due to space reasons, this article
cannot review all current CEUS applications in almost all systems (Mao et al.
2010). Breast cancer response to chemotherapy, carotid plaque characterisation,
aortic aneurysms and endoleaks assessment, musculoskeletal, pleural,
gynaecological, biliary tree applications and joints inflammation imaging,
inflammatory bowel disease activity estimation and complications in Crohn’s
disease, endoscopic use to characterise internal vasculature and to
differentiate benign from malignant pancreatic masses are some other CEUS uses.
Besides intravenously, US contrast agents are administered orally or
endocavitarily for assessing percutaneous drainage procedures (Mao et al. 2010;
Ignee et al. 2012), abscesses, pancreatitis complications, intestinal or other
fistulas, gastrooesophageal reflux and GI lumen stenoses Piscaglia et al.
2012). It should be noted that current on-label radiological indications in
Europe include only liver, breast and vascular imaging. For all other applications,
informed consent by the patient is needed. Disappointingly, in the USA
ultrasound contrast agents are still being used only for cardiology studies.

