Prostate cancer remains one of the most prevalent malignancies among men, with early and accurate detection being essential to improve treatment outcomes. Advances in deep learning have led to the development of diagnostic models capable of matching or even surpassing radiologists in certain tasks. These systems typically require large, diverse datasets to achieve robust performance, but this dependency creates challenges for adoption in clinical practice. Imaging protocols, scanner types and patient populations vary between institutions, producing what is known as domain shift. This can significantly reduce the accuracy of models trained in one environment when they are deployed elsewhere.
Addressing this issue often involves collecting and annotating extensive local datasets, which is resource-intensive and, in many cases, unrealistic for healthcare providers. An alternative is to use few-shot learning — a technique that allows models to be fine-tuned with only a small amount of locally sourced data. By leveraging this approach alongside transformer-based architectures and pretrained weights, it becomes possible to build effective diagnostic tools without the prohibitive demands of large-scale data collection. A recent implementation of such a strategy focused on multiparametric MRI for prostate cancer detection, achieving results that demonstrate both clinical feasibility and potential for broader application.
Must Read: Optimising Prostate Cancer Diagnosis with MRI-Based Risk Stratification
Addressing Domain Shift through Limited Data Training
Domain shift is a persistent obstacle in medical imaging AI. Even highly accurate models can fail when applied to data from a different institution, as variations in acquisition parameters, magnetic field strengths, patient demographics and scanner calibration can all influence image characteristics. Retraining or fine-tuning a model for each new environment is a logical solution, but traditional methods require hundreds or thousands of cases, creating a bottleneck for smaller facilities.
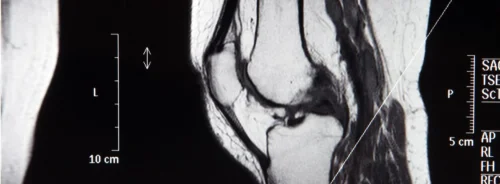
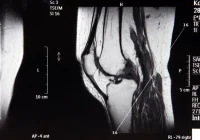
Few-shot learning offers a way forward. Instead of relying on comprehensive datasets, this method adapts existing pretrained models using a minimal number of representative local cases. In one application, just 20 cases of biopsy-confirmed prostate cancer were used for training, with an additional five for validation and a larger set for testing. A 2D transformer architecture was chosen to make the most of slice-level information from multiparametric MRI, including T2-weighted images, diffusion-weighted images and apparent diffusion coefficient maps. By stacking these sequences into a three-channel input and labelling slices individually according to pathology reports, researchers maximised the training value of each case while keeping annotation demands low.
This strategy has several advantages. It reduces the need for time-consuming segmentation by using binary slice-level classification, it limits the number of cases required to achieve useful results and it enables rapid deployment in different environments. Facilities can retain control of their own data and adapt models without the legal and logistical complexities of large-scale data sharing.
Performance Benchmarks against Radiologists
Evaluating AI systems against human experts is essential to determine clinical relevance. In this case, the few-shot learning model was compared to two experienced radiologists interpreting the same cases. Using performance measures such as the Matthews correlation coefficient and F1 score, the model achieved results close to one radiologist and slightly below the other. While the model’s recall was lower than that of the higher-performing radiologist, its precision was extremely high, indicating that when it identified cancer, it was correct in almost all cases.
The alignment between the model’s focus and clinically relevant anatomical structures was confirmed through gradient-weighted class activation mapping. This visualisation showed that the model concentrated attention on the prostate gland, particularly in regions of interest consistent with radiological assessment. Such interpretability is important for building trust in AI tools among clinicians.
The performance achieved with only 20 training cases is significant given that many of the included cases were diagnostically challenging, requiring biopsy after persistent suspicion of malignancy. This suggests that few-shot learning could be applied in real-world scenarios where only limited local data are available, without sacrificing diagnostic reliability.
External Validation and Architectural Insights
Testing the model on an external dataset, the Prostate158 collection, provided insight into how few-shot learning strategies perform under different conditions. The validation confirmed that pretraining on large, general datasets such as ImageNet substantially improves performance. Across all architectures tested, pretrained models outperformed those trained from scratch. On average, pretraining improved the area under the receiver operating characteristic curve by more than 0.17 and the precision-recall area by 0.136 at the study level.
Architectural choice was another decisive factor. Transformer-based models, including Swin Transformer, DeiT-Small and ViT-Small, consistently outperformed conventional convolutional neural networks. These architectures appear better suited to making efficient use of limited training data, likely due to their ability to capture long-range dependencies in image features. Hybrid architectures such as MaxViT also performed well, though not at the level of the top transformer models. CNN-based designs, by contrast, achieved significantly lower results in the few-shot context.
Loss function choice produced more mixed results. Binary cross-entropy remained a reliable option for most pretrained models, though focal loss occasionally offered benefits for certain transformer architectures by addressing class imbalance. The results indicate that model configuration should be tailored not only to architecture but also to the specific balance of classes in the training data.
Few-shot learning combined with pretrained transformer-based architectures presents a practical solution to one of the key barriers in deploying AI for medical imaging — the challenge of domain shift. By enabling high diagnostic performance with minimal local data, this approach reduces both the time and resources required for model adaptation while allowing facilities to maintain control over their own datasets.
In prostate cancer detection from multiparametric MRI, such a system achieved performance comparable to that of an experienced radiologist, with external validation confirming its robustness across datasets. The emphasis on transformer architectures, effective use of pretraining and careful design of training strategies provides clear guidance for institutions seeking to adopt AI tools under constrained conditions.
Looking ahead, applying these principles to other imaging tasks, expanding beyond classification into segmentation and detection, and exploring domain adaptation techniques could further enhance performance and generalisability. The ability to build clinically relevant models from as few as 20 cases has important implications for increasing access to AI-driven diagnostics, especially in resource-limited healthcare settings.
Source: Journal of Imaging Informatics in Medicine
Image Credit: iStock