HealthManagement, Volume 2 - Issue 2, Winter 2008
A major aim of health policy in most EU member countries is to regulate and control the price of, access to and the use of new and expensive medical technology. Despite this common objective, there are great differences in the means used to achieve it, because healthcare, pricing and reimbursement systems as well as domestic industry and economic status and priorities are different across countries.
This article classifies and presents the common practices and mechanisms employed in different member states to determine access to and use of new pharmaceutical and medical technology. Practices either aim at the supply side or the demand side of the medical market.
Supply-Side Practices
A commonly used measure is price control or regulation of medical
technology. Prices may be set on the basis of production costs, the prices in
other countries, of similar products within the country, the medical and economic benefits and the cost effectiveness of
therapies under consideration. Another mechanism involves the direct control of
healthcare expenditure where discounts, freezes, cuts and rebates are imposed
on manufacturers. Often, agreements about price, volume and risk sharing are in
place, where manufacturers pay back the state in cases where consumption is
greater than a certain predefined level, or in cases where medical and economic
benefits promised are not realised in real life. Other mechanisms involve the
regulation and control of industry profit rates and tax obligations. Another
group of measures involves the reimbursement policies applied. Often positive
or negative lists, reference price systems and economic evaluations are employed
to decide how to reimburse new pharmaceutical and medical technologies (see
table 1).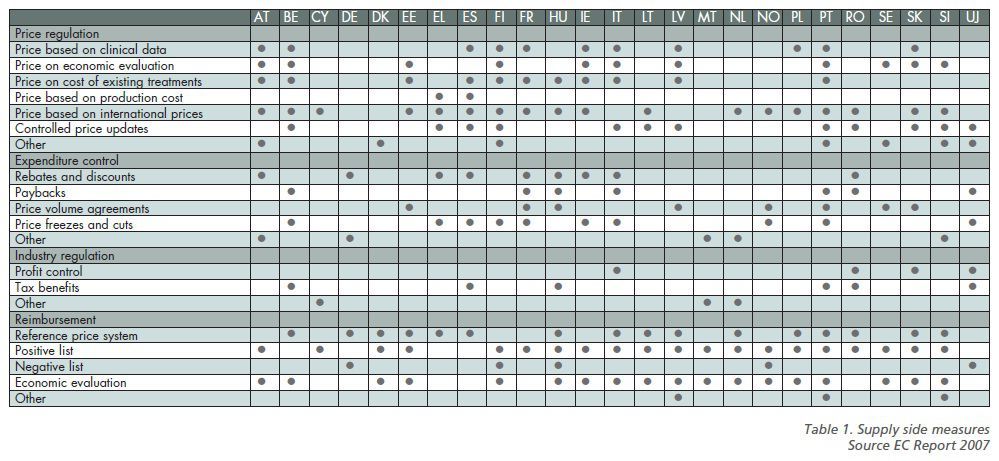
Demand Side Measures
Demand side measures focus mainly onchanging the behaviour of
the partiesdetermining the demand for
healthcaretechnology including
physicians, pharmacistsand patients. Physician
measuresinclude the implementation
of practiceand prescription
guidelines, the implementationof education, the provision
of information, the monitoring of prescribing patterns, prescription quotas,implementation of budgets
and financial incentives, all of which influence their choices and market
behaviour. In termsof patient measures the
main one includescost sharing, either in the
form of fixedor variable co-payments,
co-insurance or deductibles. Lately, information and educational campaigns aim
to guide anddefine patient behaviour.
In terms ofpharmacists, various
incentives, schemesand discounts are used to
promote substitutionof expensive with cheaper
drugs andtechnologies (see table 2).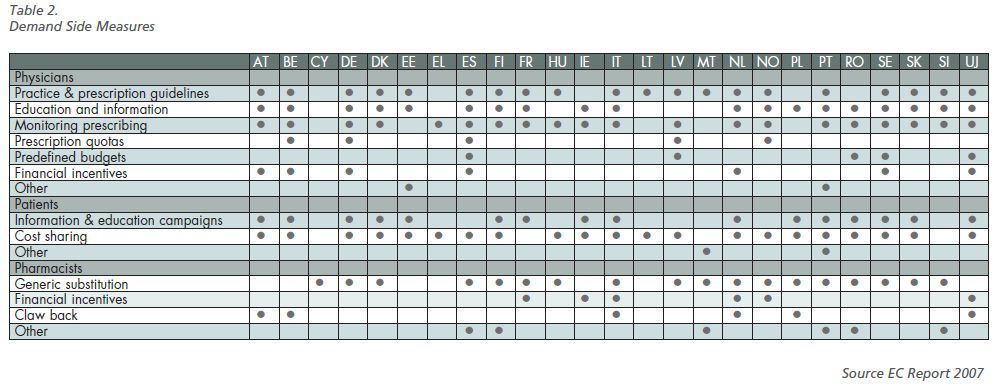
Direct Price Control
Price control is the easiest and oldest measure aiming to limit
private and public pharmaceutical and medical technology expenditure to ensure
the affordability of patient treatment and the financial sustainability of the
healthcare system. Price control is common for instance in countries such as
Austria, Finland, France, Italy, Ireland, Latvia, Lithuania, Poland, Slovenia
and Spain. Evidence suggests that where prices are not controlled, they may be
higher compared to countries where there is more regulation.
Cost Sharing
Cost sharing is a commonly used approach to control access and
expenditure even though it disproportionately affects low-income individuals. A
form of splitting the cost of healthcare services in order to reduce public
expenditure on the service, it aims to generate income or reduce expenditure
for the third-party payer, to reduce administrative costs, to make users
cost-conscious, to promote competition, to reduce abuse and inefficiency and to
facilitate access where needed. Countries where this approach is used include
Austria, Italy, UK, Belgium, France, Greece, Estonia, Finland, Latvia,
Lithuania, Poland, Portugal, Slovakia, Slovenia, Spain, Cyprus, Germany,
Norway, Denmark, Sweden and Ireland.
Reference Pricing
International price comparisons and reference pricing represent a
new trend in EU countries. Price comparisons are used to set the price of the
product in one country on the basis of other countries selected for this
purpose. Reference pricing represents a mechanism for establishing a maximum
level of third-party financing/ reimbursement for a group of products
classified somehow with a therapeutically equivalent class.
A price above the reference price is borne by the consumer to
generate and reinforce price competition and to reduce cost especially when
generic products become available, while maintaining standard quality. To be
implemented, products are first clus manufacturers tered in groups and then a
formula is used to set the reference price.
Risk-Sharing Mechanisms
Payback and risk-sharing mechanisms control access and
expenditures on new therapies. In this context, manufacturers are asked to
return a certain part of their revenue to a purchaser or the state authority if
sales exceed a previously determined maximum amount or in cases where medical benefits
have not been realised in real life either at individual or at population
level. It is used in countries like the UK, Spain, France and Norway to reduce
deviations from a prospectively set budget and to guarantee that a fair
relationship will be attained between expenditure and benefit and to reduce the
risk for the payer.
Prescribing Incentives
Prescribing incentives involve the application of various explicit
or implicit measures, which guide the choice and use of medical and
pharmaceutical technology. The objectives tend to vary depending on the aims
and priorities of those who establish them. Incentives are mainly aimed at
maximising effectiveness and minimising risk and cost.
Use of Generic Equivalents
Generic use promotion is a priority in many countries. Measures include fast track and cheaper registration of generics, encouraged or mandatory prescribing by active pharmaceutical ingredient, incentives for substitution in favour of generics by physicians and pharmacists and consumers, selective financing of generics in positive lists, reference price systems, procurement by tendering, and favourable pricing policies. Obviously the main purpose of generic policies is to increase competition and access and to contain expenditure, without compromising quality and therapeutic equivalence.
Fast-track registration and/or lower registration fees are used in
Austria, Finland, France, Hungary, Italy, the Netherlands, Portugal, Slovakia,
and Sweden. Financial or other motives for doctors are provided in the UK, the
Netherlands, Portugal, Romania, Italy and price control of generics is used in
Austria, Cyprus, Finland, France, Hungary, Ireland, Italy, Portugal, and
Slovenia. Generic substitution by the pharmacist is encouraged or imposed in
Cyprus, Denmark, Italy, Sweden, Finland, France, Hungary, Malta, Romania
Slovakia, Slovenia and the Netherlands.
Economic Evaluation
Finally, economic evaluation or otherwise cost-effectiveness analysis
is also used in many countries either to set the price or the reimbursement
level or to determine the prescription pattern of new technologies. This
approach compares the extra cost and benefit of new products in relation to existing
ones, to find out whether a fair premium is asked for the innovation. Nonetheless,
it needs to be said that it raises issues as to what the threshold of fairness may
be from one country to another.
Conclusions
EU countries are all trying to promote greater and more equitable access to new medical and pharmaceutical therapies, but they also share common concerns about limiting public expenditure. The mix of measures employed is determined by their economic and industry status and by the characteristics of their health policy and health care system.
In this context direct price regulation, international price comparisons, economic evaluation, cost sharing, reference pricing, generic use, rational prescription and pay back policies are used in different ways to achieve the above often conflicting objectives.