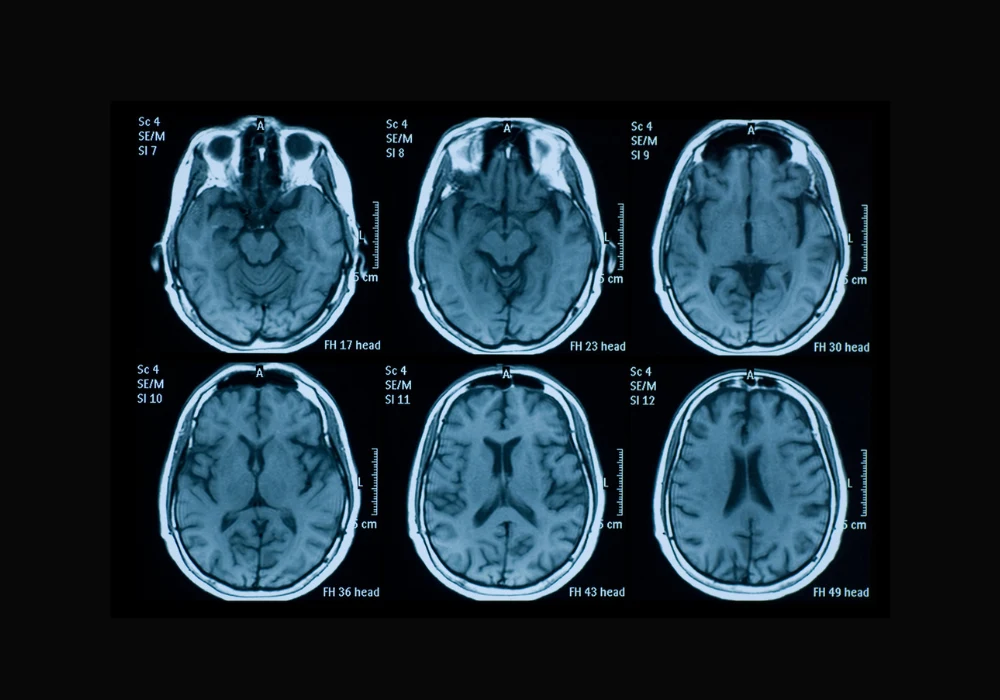
Acute ischemic stroke (AIS) remains a major cause of death and long-term disability. Timely imaging and accurate analysis of brain lesions are critical to predicting functional recovery and guiding clinical decision-making. Traditionally, infarct size has served as a principal radiologic marker, but it offers only moderate predictive power, especially in cases involving small infarcts. Chronic brain changes, such as white matter hyperintensity (WMH), add complexity to outcome prediction but are inconsistently quantified in practice. Addressing these challenges, a multilabel deep learning segmentation model has been developed to analyse acute and chronic brain lesions using multisequence MRI. This model aims to enhance short-term prognosis prediction after AIS by integrating radiologic features with clinical variables.
Automating Lesion Analysis with Deep Learning
Manual segmentation of stroke lesions is laborious, subject to inter-reader variability and limited in scalability. The newly developed convolutional neural network (CNN), based on the SegResNet architecture, enables automated multilabel segmentation of both acute infarcts and chronic WMH from multisequence MRI inputs, including FLAIR, diffusion-weighted imaging (DWI) and ADC maps. The network demonstrated strong segmentation accuracy across training, validation and testing datasets, with Dice coefficients of up to 0.95 for infarct core and 0.92 for WMH. This performance suggests the model's capability to distinguish between overlapping hyperintensities, a task that often confounds manual analysis. By providing detailed quantification of infarct volumes and WMH distribution, the model offers a more nuanced view of cerebral damage, which is crucial for comprehensive outcome prediction.
Combining Clinical and Radiologic Data for Prognostic Precision
Beyond segmentation, the study developed predictive models integrating extracted radiologic features with clinical variables such as stroke severity (NIHSS score), history of diabetes, past stroke events and treatment type. Using least absolute shrinkage and selection operator (LASSO) regression, the most relevant features were selected for outcome modelling. The resulting support vector machine-based prognostic model achieved an area under the ROC curve (AUC) of 0.81 and 0.77 in two external test datasets, outperforming models based on either infarct core volume or clinical data alone. Notably, deep WMH emerged as a consistent predictor of unfavourable outcomes, particularly in patients who received thrombolysis. Mediation analysis revealed that DWMH influenced clinical outcomes both directly and indirectly through its effect on infarct core volume, emphasising its potential as a treatment-relevant biomarker.
Must Read: Optimising Multimodal Prompts for GPT-4V in Brain MRI Diagnosis
Clinical Implications and the Role of Deep WMH
The study's findings highlight the importance of integrating chronic lesion assessment into stroke prognosis frameworks. While infarct core size has long been a primary imaging marker, the additional contribution of WMH—especially in deep brain regions—provides meaningful stratification of risk, particularly in thrombolysis-treated patients. Deep WMH accounted for significant variance in functional outcomes, with both direct and indirect effects identified through mediation analysis. These associations were not seen in patients treated with antiplatelets or anticoagulants, suggesting treatment-specific interactions. The model's predictive capability suggests it may support personalised treatment planning, especially in older patients or those with substantial chronic brain changes. By quantifying WMH burden precisely and reproducibly, this deep learning approach may guide clinicians in balancing risks and benefits when considering reperfusion therapies.
The multilabel segmentation model presents a clinically promising tool for AIS management. By accurately identifying and quantifying both acute and chronic brain lesions, it enables a more detailed and data-driven prognosis of short-term recovery. The integration of radiologic and clinical variables significantly enhances predictive performance, with deep WMH serving as a critical determinant of outcome in thrombolysis patients. These findings underscore the value of comprehensive lesion analysis in stroke care and support the adoption of AI-based tools to inform therapeutic strategies and resource allocation. Further validation in prospective studies could solidify the model’s role in routine clinical workflows and long-term stroke outcome prediction.
Source: Radiology: Artificial Intelligence
Image Credit: iStock