Magnetic resonance imaging guides prostate cancer diagnosis, yet availability, variability, workflow and cost can limit its use. Multiparametric ultrasound (mpUS) supported by artificial intelligence offers a different route that may be easier to implement at scale. A three-dimensional mpUS model was evaluated for its ability to localise clinically significant prostate cancer (csPCa) where it matters for care: at patient level. Surgical whole-mount histopathology served as a reference where available, while negative imaging or biopsies informed controls. The model’s output was converted into simulated targeted biopsies to reflect how AI-guided sampling might work in practice. Internal validation and independent external testing were used to judge whether detailed image localisation can translate into actionable guidance for clinicians.
Automated Imaging and Patient-Level Assessment
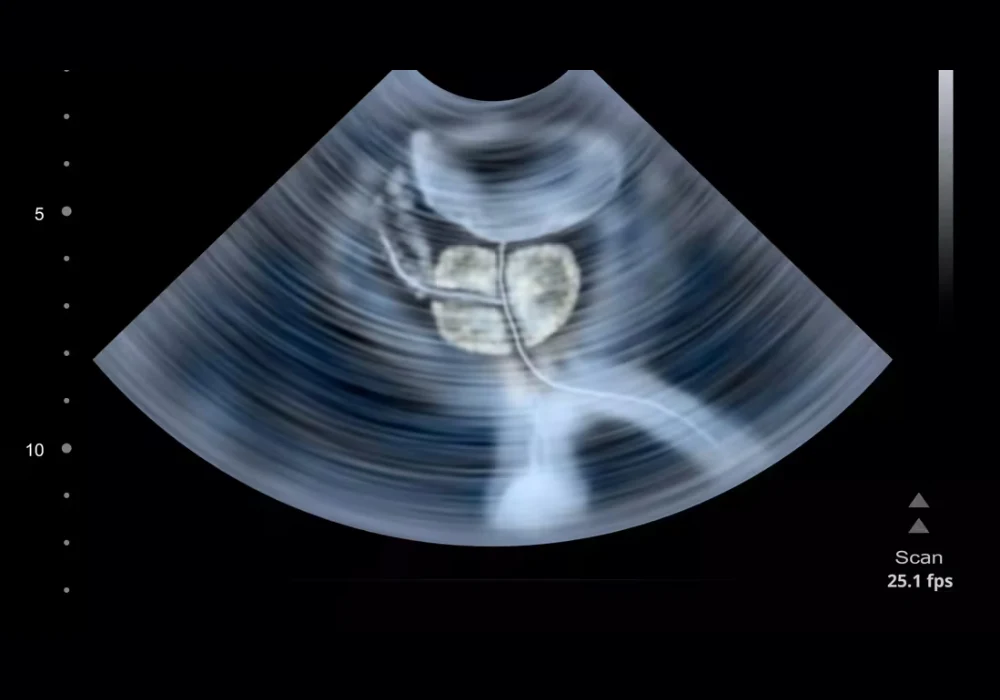
The AI model processes 3D mpUS acquired with automated protocols that combine conventional greyscale ultrasound, contrast enhancement and elastography. Patients with biopsy-proven disease who were scheduled for radical prostatectomy (RP) formed the positive reference set because whole-mount histopathology could map tumour extent. Men without csPCa were identified through low-suspicion magnetic resonance findings or negative systematic biopsies, creating a pragmatic control group aligned to real diagnostic pathways.
Must Read: Reducing Unnecessary Biopsies in Prostate Cancer Detection
Evaluation took place in two stages to support independence and reduce the risk of overfitting. First, internal performance was assessed within a development cohort using cross-validation. Second, external performance was tested in a separate cohort enrolled after training ceased, ensuring that later patients did not influence model parameters. This structure mirrored clinical translation, asking whether an algorithm trained on one group behaves similarly when confronted with new patients in a different time window.
To approximate clinical behaviour, the outputs from the AI model were turned into simulated targeted biopsies. Heatmaps highlighting suspicious regions were used to select a small number of top-ranked targets per patient, reflecting how a proceduralist would prioritise sampling in a session with limited cores. A standard transperineal path was assumed to calculate whether a hypothetical needle pass would intersect a suspicious region. These steps provided a consistent patient-level framework without requiring live procedures, making it possible to compare internal and external results on a like-for-like basis.
Simulated Targeting and Consistent Results
Across development and test cohorts, the simulated targeting approach showed that AI-guided mpUS could localise csPCa at patient level with behaviour that was stable between internal and external evaluations. Detection was strong, particularly for more aggressive disease, while specificity was more modest, consistent with a pathway in which many men had already been selected for surgery or had high suspicion on prior imaging. Predictive performance reflected this clinicopathological context and the way targets were chosen from model heatmaps.
Crucially, the external cohort behaved in line with the internal validation, indicating that the model’s localisation capability is not confined to the data on which it was built. Selecting only a couple of top-ranked targets per patient, rather than exhaustively sampling every highlighted area, still delivered a coherent pattern of results. This aligns with procedural realities, where time, anaesthesia and patient comfort constrain the number of cores and favour precise targeting over breadth.
The composition of the cohorts also reflected routine practice shaped by magnetic resonance imaging. Many men proceeding to RP had prior high-suspicion findings, while the control pathway relied on low-suspicion imaging or negative systematic biopsies. Within this mix, AI-derived targets provided a reproducible way to prioritise sampling, turning continuous probability maps into discrete actions. Taken together, these observations suggest that image-level localisation can be harnessed to support practical, patient-level decisions when translated into a small set of biopsy instructions.
Generalisability and Practical Considerations
Consistency between internal and external cohorts supports generalisability beyond the development environment, but important caveats apply. The cohorts were not representative of a pre-biopsy screening population because the prevalence of csPCa was high. That context influences all diagnostic metrics and should temper direct comparisons with settings where prevalence is lower. Moreover, the assessment relied on simulation rather than live procedures. Real-world performance can be affected by registration accuracy, prostate motion, needle trajectory and operator technique, none of which are fully captured by simulated passes.
Another limitation is the absence of a head-to-head comparison with magnetic resonance pathways within the same cohort. Many men reached surgery through MRI-guided routes, which introduces pathway bias and limits the ability to define how mpUS-based targeting would perform if used earlier or in place of MRI. Formal comparative trials are therefore needed to determine where AI-guided mpUS fits alongside existing diagnostic algorithms and how it could be sequenced with, or substituted for, MRI in different clinical scenarios.
Despite these constraints, the overall framework was rigorous. Whole-mount RP histopathology anchored the reference standard for men undergoing surgery, while negative imaging or negative systematic biopsies grounded specificity in controls. Prior image-level analyses showed strong localisation, and the present patient-level evaluation indicates that such precision can be translated into actionable targets when a small number of cores must be chosen. Beyond accuracy, questions of operational fit and cost will be important, particularly in settings where MRI access is constrained or where ultrasound-based pathways are being considered for scale.
An AI model built on automated 3D mpUS localised clinically significant prostate cancer at patient level and maintained consistent behaviour across internal validation and independent external testing when assessed through simulated targeted biopsies. Detection was strong, especially for more aggressive disease, while specificity was more modest in a pathway enriched for surgical candidates. These findings support prospective trials to confirm performance in live procedures, clarify positioning relative to MRI-guided pathways and evaluate operational and economic implications for healthcare systems.
Source: European Radiology
Image Credit: iStock