ICU Management & Practice, Volume 24 - Issue 1, 2024
The purpose of this review is to discuss the role of critical care pharmacists on the interprofessional healthcare team in the care of critically ill patients and explore current gaps in the provision of comprehensive medication management.
Introduction
“The patient’s neurological exam was concerning, new fixed and dilated pupils and an absent cough reflex, so the team wanted to initiate conversations about withdrawal of care, but I remembered that we had used a neuromuscular blocker for a procedure an hour before, and it was probably still hanging around”.
"She had come in for toxic epidermal necrolysis secondary to cefepime, and she'd been with us for three months recovering. One day, she had new fever and an increased oxygen requirement concerning for a new pneumonia, and the team ordered cefepime".
“We had acutely managed her antineutrophil cytoplasmic antibody vasculitis with plasmapheresis, steroids, and cyclophosphamide, and she was finally doing better. She was having hypertension that the team wanted to manage, and the intern ordered hydralazine, but her initial vasculitis presentation had been triggered by hydralazine”.
“I received an order for 20 mg every 4 hours of intravenous morphine, which didn’t seem right. Turns out someone had plugged in numbers incorrectly into an online calculator, and they actually wanted 2 mg morphine”.
“The patient was scheduled to receive prothrombin complex concentrate before going to the operating room, but I noticed that it was scheduled to be given more than twelve hours before the surgery and knew it probably wouldn’t be effective in that time window”.
“I walked by a room and saw methylene blue hanging (it’s pretty distinctive, hard to miss). When I asked why the patient was supposed to be receiving it, I found out that the team had actually wanted meropenem”.
“A patient with acute acetaminophen overdose was in the ICU for monitoring, receiving intravenous acetylcysteine. I went to discuss the titration with the nurse and found that the line was clamped – the acetylcysteine had been charted but wasn’t running”.
The story of the critical care pharmacist is one of counterfactuals, the "what ifs" of critical care medicine. Critical care pharmacists do not perform lifesaving procedures, and they do not generally actively hold a patient's life in their hands. Ask any critical care pharmacist, no matter how experienced, and they will struggle to pinpoint an action or a moment in their career that definitively saved someone's life. Yet the stories are there, catalogued as "interventions" and "near-misses," the "but-fors” that speak to negative outcomes prevented. The anecdotes may be jarring, and there is an immediate impulse to push them away as just that: anecdotal, one-off, idiosyncratic, Swiss cheese model, or negligent. Of course, we would never be the ones to have such an error of commission or omission. Yet, it is that same style of thinking, full of cognitive shortcuts, that makes us so quick to add a new medical intervention or technology to practice while still neglecting to consistently wash our hands before entering a patient's room, despite knowing this undoubtedly saves lives (Kahneman et al. 2021; Klotz 2021; Pascale et al. 2010).
Wisdom is knowing how little we know, and humility is knowing we are fallible. Medications have incredible power to heal and harm (Ely 2021; Sikora 2023). While necessary and lifesaving, they are also complex and dangerous, twin currents for a perfect storm. (Kane-Gill 2012) The intensive care unit (ICU) can be a particularly dangerous place for patients, a setting where a high probability of error meets low tolerance for that error (Cullen 1997; Halpern et al. 2016; Maslove et al. 2017; Practices 2018). Indeed, ICUs are places where cognitive load is high, decisions are frequently made with inadequate information, and risks from incorrect decisions are higher than in other care environments. Medications are stark cases in point for this reality: patients in the ICU receive, on average, twice as many medications as ward patients (Sikora 2023) and that increased volume means patients are more than twice as likely to experience an adverse drug event (ADE) (Maslove et al. 2017; Practices 2018).
As such, the best ICU teams know that ‘none of us can know everything.’ They leverage multi-professional expertise to make the best decision every time given the circumstances of an information-rich environment under time pressure, incorrect or missing information, and a high cognitive load coupled with decision-making heuristics and cognitive biases we use to lighten that burden. It is this acknowledgement and exploration of our limits that informs international efforts like the "Choosing Wisely in Critical Care" campaign from the Society of Critical Care Medicine and the American Board of Internal Medicine, which advocate for systems of thought that account for the fallibility of human judgement, particularly noting that 'less can be more'. Moreover, the best ICU teams respect that medications are causal agents, for good and bad outcomes alike, and take intentional steps to maximise those benefits while minimising the risks (Sikora 2023). The best available evidence, which included studies conducted across the globe, supports the kernel of truth in those stories: a critical care pharmacist on rounds with the ICU team, performing comprehensive medication management, reduces adverse drug events by nearing 70% and odds of mortality by 20% (Leape et al. 1999; Lee et al. 2019). Pharmacists save lives.
Yet not every critically ill patient has a critical care pharmacist (Borthwick et al. 2018; MacLaren et al. 2021; Newsome 2020a; Pedersen et al. 2019) This is true in at least 30% of ICUs in the United States (U.S.) and the United Kingdom (U.K.), and even in those settings where a pharmacist is present, a high workload can preclude optimal patient care. Weekend rounding services are rare in both U.S. and U.K. studies (Borthwick et al. 2023; Newsome et al. 2021; Sikora 2023; Sikora et al. 2022; Sikora and Martin 2022; Smith et al. 2021). Improving patient access to critical care pharmacists has great potential as a high-yield quality improvement endeavour. Here, we discuss frames of mind that cause us to neglect the vital importance of medication optimisation and those who specialise in this endeavour with the goal to guide discussions for how best to improve patient-centred outcomes.
Standard of Care and Why Pharmacists Are Included
In critical care medicine, a small number of interventions are carried forward as standard of care. These interventions, though frequently unflashy and almost the antithesis of precision medicine, form the backbone of ICU care, the lowest common denominator for all patients. What makes these stand out against the myriad other potential interventions in critical care medicine? First, they represent relatively small changes. Setting a ventilator to deliver a smaller tidal volume or standardising resuscitation practices, while paradigm shifting and requiring education, are not resource intensive (ARDSNet et al. 2000; Rivers et al. 2001). Second, they impact a significant proportion of patients admitted to the ICU. Mechanical ventilation is required by 20-40% of all adult ICU patients (Levy et al. 2018). Sepsis affects nearly 2 million patients annually in the U.S. and is the leading cause of death (SCCM 2024). Third, in the context of the relatively low cost of implementation and broad application (small changes done often), these interventions have an outsize impact on patient outcomes. These interventions revolutionised supportive care practices because they routinely and uniformly reduced mortality when broadly applied to common ICU admission diagnoses that are notoriously recalcitrant to disease-targeted therapy.
Medications are another lowest common denominator in ICU care. Every patient in the ICU receives medications. In fact, ICU patients are prescribed an average of >20 medications, with many deemed high risk by the Institute for Safe Medication Practices (Maslove et al. 2017; Practices 2018). Medication use has taken tremendous strides in the domain of safety over the last 50 years: computerised provider order entry (CPOE), barcoding, dose-checking software, and smart infusion pumps make the operational side of giving drugs to patients safer. Yet, as Peter Drucker quips, “There is nothing so useless as doing efficiently that which should not be done at all”. All those technologies make one key assumption: that the medication (including dose, frequency, route, formulation, etc.) currently being administered to the patient was truly the most correct, the safest and most optimal medication for that individual patient and their disease state. Answering this question in a nuanced, evidence-based manner is the domain of the critical care pharmacist.
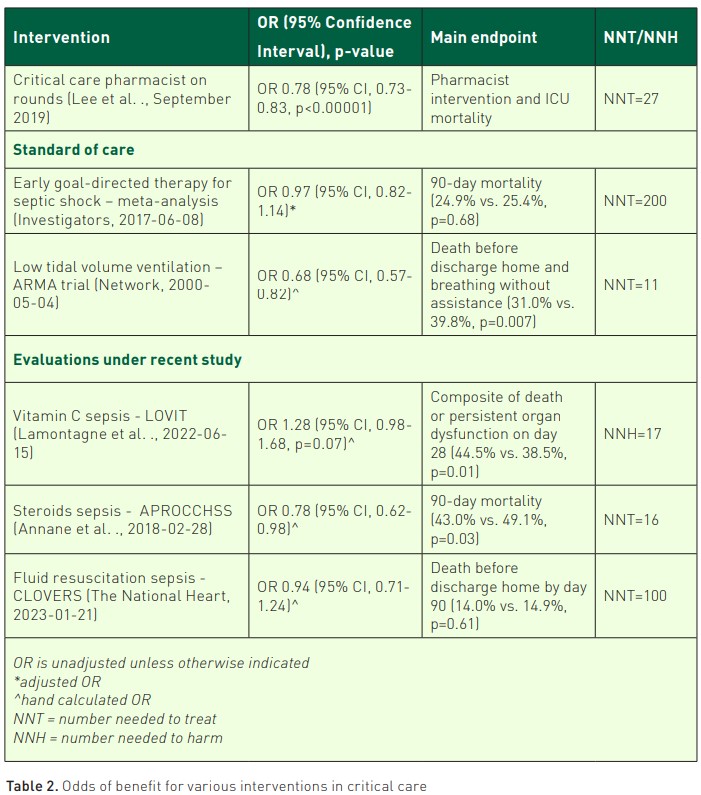
Indeed, for all the ‘standard of care’ interventions that come to be codified in the likes of FASTHUGS (Vincent 2005), estimates indicate that unintended ADEs occur in 5% of the over 36 million hospitalised patients per year, and unintended sentinel events occur at a rate of 38.8 events per 100 patient days in the ICU (Cullen et al. 1997; Halpern et al. 2016; Valentin et al. 2006). Each individual ADE doubles patient mortality, and the annual costs of treating ADEs exceeds $1.5 billion in the U.S. alone (Cullen et al. 1997; Halpern et al. 2016; Kane-Gill et al. 2010; Kane-Gill et al. 2012; Kaushal et al. 2007; Maslove et al. 2017; Practices 2018). Similar U.S. estimates show approximately 1.8 million ADEs in hospitalised patients, with estimates of 9,000 patients that die as a direct result of a medication error per year with an expected cost of $40 billion in relation to medication errors (Tariq 2023). In a recent study, two pharmacists identified over 600 medication errors in an eight week study period, despite working in an academic medical centre with safety advents of barcoding, CPOE, etc. (Chase et al. 2023). A large evaluation in the U.K. found that 1 in 6 medication orders required pharmacist intervention (Rudall et al. 2017). This finding has been observed in two other significant studies showing nearly 70% reductions of ADEs by critical care pharmacists on rounds (Leape et al. 1999; Lee et al. 2019). Presence on rounds as part of the team appears important for meaningful impact in studies from the U.S., U.K., and France (Bourne et al. 2022; Bourne et al. 2017; Leguelinel-Blache et al. 2018; Smetana et al. 2023). Table 1 provides a brief review and associated literature for the profession of critical care pharmacy. Critical care pharmacists promote a culture of evidence-based medication use that supports optimal patient-centred outcomes.

Yet, such cognitive services risk being what Arlene Kaplan Daniels described as 'invisible work' – that which goes unacknowledged and unregulated but no less essential to outcomes. Critical care pharmacists on diverse, multi-professional teams have been repeatedly shown to improve patient-centred outcomes (Pedersen et al. 2018; MacTavish et al. 2019; Stollings et al. 2018). When placing the role of pharmacists in the context of well-known ICU paradigms or other trends of study, this service is essential (as stated by the endorsed Position Statement on Critical Care Pharmacists) (Lat et al. 2020). Table 2 provides a summary of ICU paradigms.
Pharmacists in the ICU are not revenue generators, and the inability to bill for cognitive services, as well as the awkward pairing of pharmacist salaries and drug costs in pharmacy department budgets, leads to complicated conversations surrounding the implementation of this essential resource. While addressing some of these structural issues in the future could change the conversation around the economics of pharmacy resources, under current systems, adding a pharmacist to an ICU still represents a simple, resource-sparing, and ultimately cost-effective change. When studying the activities and interventions of 215 pharmacists across 85 medical centres in the U.S., the average cost avoidance to salary ratio for a critical care pharmacist was estimated to be between $3.3:1 and $9.6:1 (Rech et al. 2021). Compared to a centralised model, a decentralised model with pharmacists physically present and rounding with the ICU team was associated with over $200,000 in additional cost avoidance (Kopp et al. 2007). With pharmacy charges contributing nearly 20% of total ICU charges and ICU drug costs potentially making up nearly a third of a hospital’s overall drug budget, ICU pharmacists (and the medication therapy expertise they bring to the team) represent a resource-conservative intervention (Altawalbeh et al. 2018).
How Cognitive Biases Undervalue Medication Therapy Expertise
Inertia to change has been documented across continents (Borthwick et al. 2023; Muñoz-Pichuante et al. 2024; Sikora 2023). It can be tricky to value medication therapy expertise because such cognitive services have an element of the ephemeral, in contrast to the many "hands-on" skill sets relevant to ICU practice. Reflecting on the type of cognitive biases that can lead to such implementation inertia is an important step towards restructuring ICU teams to value the role of this skill set.
Each ICU skill set takes time and practical direct patient care experience to develop. Performing procedures – placing lines, intubating, poking needles and tubes into various spaces – requires skill development. Communicating prognoses and plans of care with patients and family members is a different but no less important skillset. Along those lines, developing a skill set for patient assessment requires years of specialised training, knowledge base development, and direct patient care experience. One must have a mental catalogue of many potential diagnoses augmented by available resources. A mental image or model of each diagnosis needs to be formed, both of characteristic findings as well as elements that would be inconsistent, to inform what to look for or what studies to order to make a particular diagnosis more or less likely. Performing a thorough physical examination is its own observational skill set, from palpating an enlarged liver to listening to lung and heart sounds. But that is only part of the equation; you also must be able to interpret labs, images, and tracings on a screen or a piece of paper. It requires pattern recognition but also an understanding of patient-specific factors that don't fit with the pattern. All the while, you are making mental adjustments to the probability of each differential diagnosis based on new data points being added to the mental model.
Medication expertise is its own unique skill set. It receives only a fraction of dedicated time in medical schools, but true expertise is developed through dedicated education and training. Critical care pharmacists provide that specialised knowledge base and skill set as active members of the ICU team. They are the members of the team best positioned to evaluate the complexities of the patient-medication system and how a drug will interact with not only the disease but also with unique patient factors and other medications the patient is receiving (Sikora 2023). They are the members best able to navigate the balance of the potential benefits of a medication for a given patient against the risks of harm. Their specialised knowledge and training, combined with a wealth of real direct patient care experience and a dedication to evaluation of emerging literature and guidelines, position them to be leaders of a culture of evidence-based medical practice in the ICU. In short, just as you would not want a pharmacist performing your thoracentesis or reading your chest x-ray, you probably would want a pharmacist having a significant say in your medication regimen (or in the medication use culture of an ICU). But if the specialised knowledge and skills that critical care pharmacists bring is so important, why are they underutilised?
When performing a patient assessment, there is no mental calculus occurring as the picture of the patient is formed; we are not adjusting the probability of one diagnosis down by 3% based on the lab value that just resulted and a study that guides that precise valuation. Rather, we utilise heuristics, mental shortcuts that allow us to process information and make decisions quickly (Kahneman 2013). These heuristics, while efficient, are not always optimal and are known to be prone to bias. Recently, there has been greater recognition and acceptance of bias in medical decision-making, and various artificial intelligence-based decision models are specifically designed to help reduce the impact of bias in human decision-making (Sikora 2023; Webster et al. 2021). Being aware of bias can help reduce the impact. Anchoring bias, for example, occurs when a patient presents looking very much like they fit a particular diagnosis, one that would tie together elements of the story neatly (Tversky and Kahneman 1974); to combat that bias, we can keep our differential broad until more data confirms a diagnosis. Base rate neglect or the availability heuristic can occur when we choose antibiotics that cover vancomycin-resistant enterococcus (VRE) in the absence of risk factors because we recently had a patient with a VRE infection who behaved similarly (Redelmeier and Tversky 1990). If we acknowledge the ubiquitous and unconscious presence of these biases, we can combat their impact on our decision-making.
The impact of bias can also be seen in the slow implementation of pharmacist services in the ICU, a number of which are highlighted in Table 3. There are several key themes, including general resistance to changing established systems or a reluctance to relinquish control over some portion of patient care to another profession.
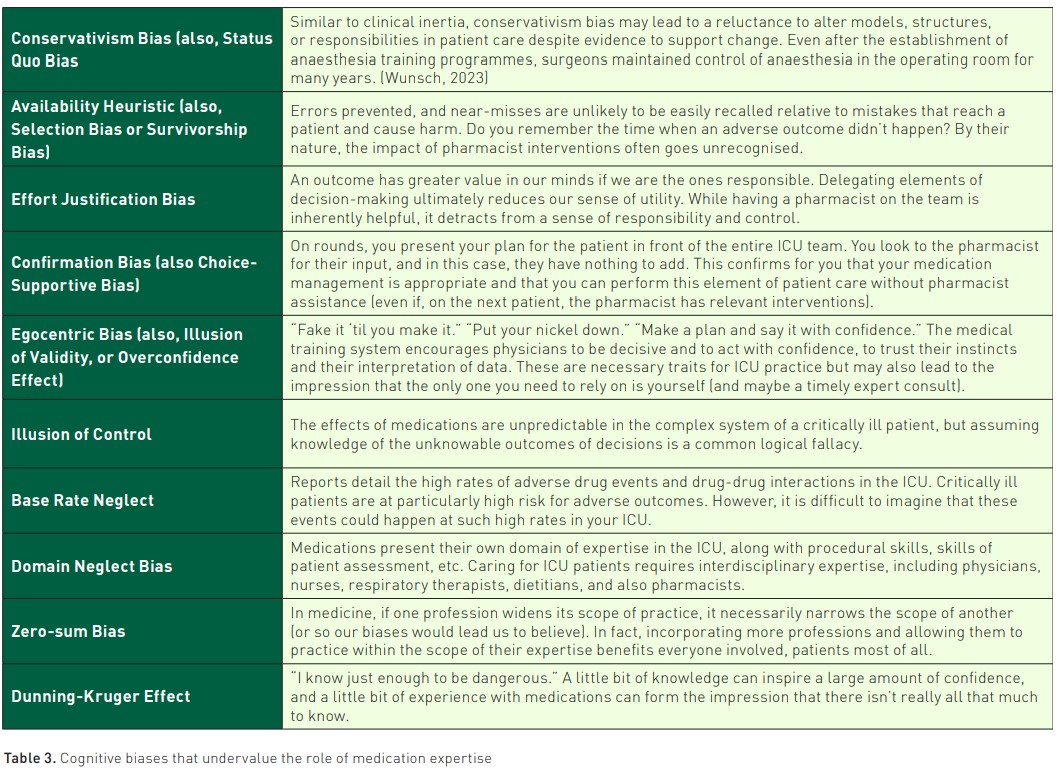
Acknowledging and naming these biases is a key first step in combatting their effects. Reframing critical care pharmacists as care extenders, members of the team that can elevate the level of practice and shift time and energy towards other high-level tasks for which individuals are specially trained may reinforce the essential nature of this resource in the ICU.
Constructs for Re-imagining Medication Use in the ICU
Beyond awareness of the cognitive biases that can cause us to neglect important information, intentionally adopting frameworks of thought regarding medication use has the potential to improve evidence-based use of medications in the ICU.
1. Medications are causal agents. We conduct randomised controlled trials with the intention to understand the causal effect of a medication on a disease state and are comfortable with relating the use of antibiotics to the resolution of an infection. Yet, other subtleties abound: what are the ramifications of the choice to go from intravenous to oral? Each time we attach a medication to a patient's IV line, there is risk of infection, fluid overload, unexpected incompatibility, etc. What is the difference between two antibiotics with similar spectrum? The difficulty of parsing these nuances often makes us push them away as irrelevant, yet in the age of Big Data, artificial intelligence, Bayesian analysis, and causal inference, we may finally have the tools to begin to refine (and optimise) our drug selection(Pearl and Mackenzie 2018).It has been previously proposed that there is Patient – Medication Optimisation – Outcome Pathway and already machine learning methods are showing novel ability to reflect the role of medications on outcomes (Al-Mamun et al. 2021; Rafiei et al. 2023; Sikora 2023; Sikora 2022; Sikora et al. 2023a; Sikora et al. 2023b; Liu 2023). To summarise this causal line of thinking (and the role of those who provide comprehensive medication management), we propose a twist on Mark Twain’s adage: The difference between the almost right medication and the right medication is really a large matter—it's the difference between the lightning bug and the lightning. Figure 1 provides a series of causal diagrams.
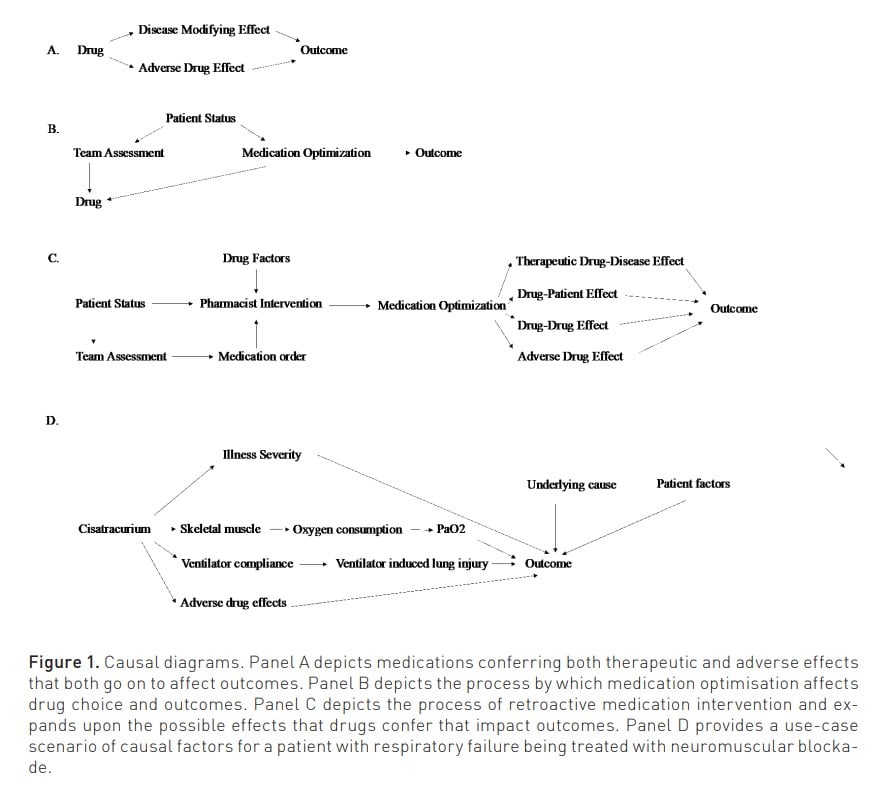
2. The Goldilocks effect of medication regimen complexity. Paracelsus stated, “All things are poison, and nothing is without poison; the dosage alone makes it, so a thing is not a poison”. Medications obviously conform to this axiom. Thinking about medication use more broadly, the aggressiveness with which medication interventions are provided can be conceptualised in a global sense as medication regimen complexity (encompassing number, type, intensity, and other factors associated with medication intervention). Indeed, a novel metric developed with the precise goal of summarising medication regimen complexity in the intensive care unit (MRC-ICU) has repeatedly shown key relationships with patient-centred outcomes (mortality, length of stay), ICU complications (fluid overload, drug-drug interactions, mechanical ventilation, medication errors), and critical care pharmacist workload (interventions, intervention intensity) (Al-Mamun 2021; Chase 2023; Gwynn et al. 2019; Newsome 2019; Newsome 2020b; Olney 2021; Rafiei 2023; Sikora 2023; Sikora et al. 2022; Sikora 2022; Sikora et al. 2023a-f; Smith et al. 2021; Webb 2021; Webb 2022; Liu 2023; ). Given a patient's condition, there is an appropriate level of medication regimen complexity needed to treat them (e.g., a patient with multi-drug resistant infection requiring multiple broad-spectrum antibiotics): to have too little complexity will likely result in death from infection but too much complexity – adding unnecessary antibiotics and aggressive interventions – will likely also lead to poor outcomes. This theoretical line of thinking has begun to be evaluated (Sikora et al. 2023a). There is a correct "dose" of medication complexity, but similar to the trends of pulmonary artery catheters and surgical intervention for necrotising pancreatitis, there is value in caution of wanton usage (Harvey et al. 2005; Mier et al. 1997; NHLBI 2006; van Santvoort et al. 2010).

Practical Application for Improving Evidence-Based Medication Use
Modifications to research infrastructure and ICU team design have potential to improve medication use.
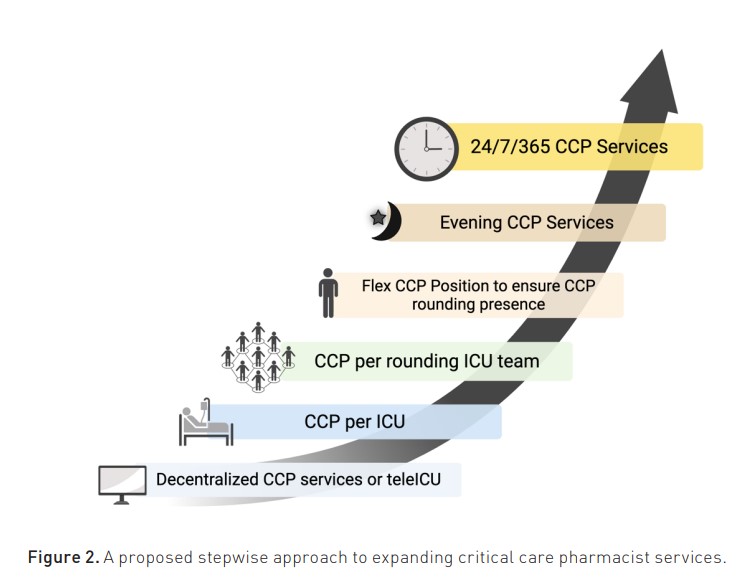
1. Stepwise improvement of access to critical care pharmacist services. A proposed vision is that every critical care team that cares for critically ill patients includes critical care pharmacists integrated into the team to provide real-time comprehensive medication management at the bedside. Indeed, an ICU patient would never be without an intensivist and dedicated nurse, and this same patient should never be without a critical care pharmacist. Interprofessional team decision-making would incorporate the specific expertise of each professional to devise a proactive treatment plan, including both medication and non-medication therapy. These recommendations (including those of a critical care pharmacist) would be appropriately documented and appropriately funded through either billable models or as essential services in the same manner as ICU nurses and other healthcare workers. Inherent to this process, critical care pharmacist minimum staffing requirements for ICU patients would be established at institutional levels but also at national accreditation levels, given the importance of healthcare professional workload to patient-centred outcomes. A potential flow for such workload redesign is provided in Figure 2, although institutional nuances must be identified such that workload allows for consistent, high-level critical care pharmacist care (Sikora and Martin 2022). Workload redesign considerations include (1) ensuring consistent rounding presence given the strong data to support its benefit to patient outcomes and (2) providing consistency in level of critical care pharmacist care both on weekdays (e.g., minimising cross coverage) and on non-weekday, daytime shift (e.g., rounding on holidays and weekends).
2. Building research infrastructure that incorporates robust medication data. To interpret even the most routine medication order, a striking number of factors must be incorporated. These have been previously proposed to fall into three main categories: (1) drug product information (e.g., drug, dose, formulation, route, frequency), (2) clinical information (e.g., mechanism of action, drug-drug interactions), and (3) medication order information, or the specific drug product in the context of that individual patient (e.g., urine output, disease, etc.). An immediate reaction to this type of complexity is to look for shortcuts: lumping drugs by body system they act upon (thus condensing cisatracurium continuous infusion and haloperidol as ‘neurology’) or to drop formulation information altogether. Yet, the difference between subcutaneous lidocaine and intravenous lidocaine is as big as the lightning bug and the lightning, between getting a cavity filled and treating life-threatening ventricular storm. Though both are technically the same chemical compound, to reduce the high dimensionality of this data is to lose vital information. The result is that very few, if any, prediction-based algorithms incorporate medication data, and nuanced clinical decision support or medication regimen safety checking is lacking. The first steps have been taken towards the development of a common data model and associated ontology for ICU medications and the development of machine learning methods suited to the management and incorporation of this vital data source, though much is yet to be done (Rafiei et al. 2023; Sikora et al. 2023d; Keats et al. 2023).
Conclusion
The complexity of management in the modern care of critically ill patients requires a healthcare team. Comprehensive medication management and those who can provide this cognitive service are essential to this team-based approach. Thoughtfully exploring the biases that can lead us to clinical inertia and ideating on needed steps are important to ensure that all patients receive optimal care.
Funding
This work was supported by the Agency of Healthcare Research and Quality [R21HS028485 and R01HS029009].
Acknowledgements
The authors thank Catharine A McKenzie, PhD FRPharmS, and Mark Borthwick, FFRPS, FRPharmS, for their valuable input.
Conflict of Interest
None.
References:
Acute Respiratory Distress Syndrome Network; Brower RG, Matthay MA, Morris A et al. (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342(18):1301-8.
Al-Mamun MA, Brothers T, Newsome AS (2021) Development of Machine Learning Models to Validate a Medication Regimen Complexity Scoring Tool for Critically Ill Patients. Ann Pharmacother. 55(4):421-429.
Altawalbeh SM, Saul MI, Seybert AL et al. (2018) Intensive care unit drug costs in the context of total hospital drug expenditures with suggestions for targeted cost containment efforts. J Crit Care. 44:77-81.
Annane D, Renault A, Brun-Buisson C et al. (2018) Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 378(9).
ASHP. Comprehensive Medication Management. Available at https://www.ashp.org/advocacy-and-issues/key-issues/other-issues/comprehensive-medication-management?loginreturnUrl=SSOCheckOnly
Borthwick M, Barton G, Bourne RS, McKenzie C (2018) Critical care pharmacy workforce: UK deployment and characteristics in 2015. Int J Pharm Pract. 26(4):325-333.
Borthwick M, Barton G, Ioannides CP et al. (2023) Critical care pharmacy workforce: a 2020 re-evaluation of the UK deployment and characteristics. Hum Resour Health. 21(1):28.
Bourne RS, Jennings JK, Panagioti M et al. (2022) Medication-related interventions to improve medication safety and patient outcomes on transition from adult intensive care settings: a systematic review and meta-analysis. BMJ Qual Saf. 31(8):609-622.
Bourne RS, Shulman R, Tomlin M et al. (2017) Reliability of clinical impact grading by healthcare professionals of common prescribing error and optimisation cases in critical care patients. Int J Qual Health Care. 29(2):250-255.
Buckley MS, Knutson KD, Agarwal SK et al. (2020) Clinical Pharmacist-Led Impact on Inappropriate Albumin Use and Costs in the Critically Ill. Ann Pharmacother. 54(2):105-112.
Chase AH, Forehand C, Keats K et al. An evaluation of medication regimen complexity’s relationship to medication errors in critically ill patients. Hosp Pharm. Accepted.
Chase AM, Azimi HA, Forehand CC et al. (2023) An Evaluation of the Relationship Between Medication Regimen Complexity as Measured by the MRC-ICU to Medication Errors in Critically Ill Patients. Hosp Pharm. 58(6):569-574.
Cascone AE, Sullivan J, Ackerbauer K et al. (2023) Pharmacist-Initiated De-Prescribing Efforts Reduce Inappropriate Continuation of Acid-Suppression Therapy Initiated in the ICU. Am J Med. 136(2):186-192.
Chaverri-Fernández JM, Zavaleta-Monestel E, Murillo-Cubero J et al. (2022) The Pharmacist's Role in the Implementation of FASTHUG-MAIDENS, a Mnemonic to Facilitate the Pharmacotherapy Assessment of Critically Ill Patients: A Cross-Sectional Study. Pharmacy (Basel). 10(4):74.
Chiang LH, Huang YL, Tsai TC (2021) Clinical pharmacy interventions in intensive care unit patients. J Clin Pharm Ther. 46(1):128-133.
Control, C. f. D. Key Terms. NHSN Healthcare Personnel Safety Component. Available at https://www.cdc.gov/nhsn/pdfs/hps-manual/exposure/6-hps-key-terms.pdf
Cullen DJ, Sweitzer BJ, Bates DW, et al. (1997) Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. 25(8):1289-1297.
Cvikl M, Sinkovič A (2020) Interventions of a clinical pharmacist in a medical intensive care unit - A retrospective analysis. Bosn J Basic Med Sci. 20(4):495-501.
Ely EW (2021) Every Deep-drawn Breath: A Critical Care Doctor on Healing, Recovery, and Transforming Medicine in the ICU. Scribner.
Gwynn ME, Poisson MO, Waller JL, Newsome AS (2019) Development and validation of a medication regimen complexity scoring tool for critically ill patients. Am J Health Syst Pharm. 76(Supplement_2):S34-S40.
Halpern NA, Goldman DA, Tan KS, Pastores SM (2016) Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000-2010. Crit Care Med. 44(8):1490-1499.
Harvey S, Harrison DA, Singer M et al. (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 366(9484):472-477.
Kahneman D (2013) Thinking, Fast and Slow. Farrar, Straus and Giroux.
Kahneman D, Sibony O, Sunstein CR (2021) Noise: A Flaw in Human Judgment. Little Brown Spark.
Kane-Gill SL, Jacobi J, Rothschild JM (2010) Adverse drug events in intensive care units: risk factors, impact, and the role of team care. Crit Care Med. 38(6 Suppl):S83-S89.
Kane-Gill SL, Kirisci L, Verrico MM, Rothschild JM (2012) Analysis of risk factors for adverse drug events in critically ill patients. Crit Care Med. 40(3):823-828.
Kaushal R, Bates DW, Franz C et al. (2007) Costs of adverse events in intensive care units. Crit Care Med. 35(11):2479-2483.
Klotz L (2021) Subtract: The Untapped Science of Less. Flatiron Books.
Kopp BJ, Mrsan M, Erstad BL, Duby JJ (2007) Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am J Health Syst Pharm. 64(23):2483-2487.
Lamontagne F, Masse M-H, Menard J et al. (2022) Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N Engl J Med. 386(25).
Lat I, Paciullo C, Daley MJ et al. (2020) Position Paper on Critical Care Pharmacy Services: 2020 Update. Crit Care Med. 48(9):e813-e834.
Leape LL, Cullen DJ, Clapp MD et al. (1999) Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 282(3):267-70.
Lee H, Ryu K, Sohn Y et al. (2019) Impact on Patient Outcomes of Pharmacist Participation in Multidisciplinary Critical Care Teams: A Systematic Review and Meta-Analysis. Crit Care Med. 47(9):1243-1250.
Lee H, Ryu K, Sohn Y et al. (2019) Impact on Patient Outcomes of Pharmacist Participation in Multidisciplinary Critical Care Teams: A Systematic Review and Meta-Analysis. Critical Care Medicine. 47(9).
Leguelinel-Blache G, Nguyen TL, Louart B et al. (2018) Impact of Quality Bundle Enforcement by a Critical Care Pharmacist on Patient Outcome and Costs. Crit Care Med. 46(2):199-207.
Levy MM, Evans LE, Rhodes A (2018) The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 44(6):925-928.
MacLaren R, Roberts RJ, Dzierba AL et al. (2021) Characterizing critical care pharmacy services across the United States. Crit Care Explor. 3(1):e0323.
MacLaren R, Bond CA, Martin SJ, Fike D. (2008) Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 36(12):3184-9.
MacTavish P, Quasim T, Shaw M et al. (2019) Impact of a pharmacist intervention at an intensive care rehabilitation clinic. BMJ Open Quality. 8(3).
Maslove DM, Lamontagne F, Marshall JC, Heyland DK (2017) A path to precision in the ICU. Crit Care. 21(1):79.
Michalets E, Creger J, Shillinglaw WR (2015) Outcomes of expanded use of clinical pharmacist practitioners in addition to team-based care in a community health system intensive care unit. Am J Health Syst Pharm. 72(1):47-53.
Muñoz-Pichuante D, Latorre M, Villa-Zapata L et al. (2024) Investigating the links among MRC-ICU, SOFA, and cost avoidance from pharmacists in a Chilean ICU. Crit Care Med. 52(1):S430.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network, Wheeler AP, Bernard GR et al. (2006) Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 354(21):2213-2224.
Network TARDS (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342(18).
Newsome AS, Smith SE, Jones TW et al. (2020a) A survey of critical care pharmacists to patient ratios and practice characteristics in intensive care units. J Am Coll Clin Pharm. 3:68-74.
Newsome AS, Anderson D, Gwynn ME, Waller JL (2019) Characterization of changes in medication complexity using a modified scoring tool. Am J Health Syst Pharm. 76(Supplement_4):S92-S95.
Newsome AS, Murray B, Smith SE et al. (2021) Optimization of critical care pharmacy clinical services: a gap analysis approach. Am J Health Syst Pharm. 78(22):2077-2085.
Newsome AS, Smith SE, Olney WJ, Jones TW (2020b) Multicenter validation of a novel medication-regimen complexity scoring tool. Am J Health Syst Pharm. 77(6):474-478.
Olney WJ, Chase AM, Hannah SA et al. (2021) Medication regimen complexity score as an indicator of fluid balance in critically ill patients. J Pharm Pract. 897190021999792.
Pascale RT, Sternin J, Sternin M (2010) The power of positive deviance: how unlikely innovators solve the world's toughest problems. Harvard Business Press.
Pearl J, Mackenzie D (2018) The book of why: the new science of cause and effect. Basic Books.
Pedersen CA, Schneider PJ, Ganio MC, Scheckelhoff DJ (2019) ASHP national survey of pharmacy practice in hospital settings: monitoring and patient education-2018. Am J Health Syst Pharm. 76(14):1038-1058.
Practices IOSM. High alert medications. Available at https://www.ismp.org/sites/default/files/attachments/2018-08/highAlert2018-Acute-Final.pdf
PRISM Investigators; Rowan KM, Angus DC, Bailey M et al. (2017) Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 376(23):2223-2234.
Rafiei A, Rad MG, Sikora A, Kamaleswaran R (2023) Improving irregular temporal modeling by integrating synthetic data to the electronic medical record using conditional GANs: a case study of fluid overload prediction in the intensive care unit. medRxiv.
Rech MA, Gurnani PK, Peppard WJ et al. (2021) PHarmacist Avoidance or Reductions in Medical Costs in CRITically Ill Adults: PHARM-CRIT Study. Crit Care Explor. 3(12):e0594.
Redelmeier DA, Tversky A (1990) Discrepancy between medical decisions for individual patients and for groups. N Engl J Med. 322(16):1162-1164.
Rivers E, Nguyen B, Havstad S et al. (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 345(19):1368-1377.
Rudall N, McKenzie C, Landa J et al. (2017) PROTECTED-UK - Clinical pharmacist interventions in the UK critical care unit: exploration of relationship between intervention, service characteristics and experience level. Int J Pharm Pract. 25(4):311-319.
Sikora A (2023) Critical Care Pharmacists: A Focus on Horizons. Crit Care Clin. 39(3):503-527.
Sikora A, Ayyala D, Rech MA et al. (2022) Impact of Pharmacists to Improve Patient Care in the Critically Ill: A Large Multicenter Analysis Using Meaningful Metrics With the Medication Regimen Complexity-ICU (MRC-ICU) Score. Crit Care Med. 50(9):1318-1328.
Sikora A, Devlin JW, Yu M et al. (2023a) Evaluation of medication regimen complexity as a predictor for mortality. Sci Rep. 13(1):10784.
Sikora A, Jeong H, Yu M et al. (2023b) Cluster analysis driven by unsupervised latent feature learning of medications to identify novel pharmacophenotypes of critically ill patients. Sci Rep. 13(1):15562.
Sikora A, Keats K, Murphy DJ et al. (2023c) A Common Data Model for the standardization of intensive care unit (ICU) medication features in artificial intelligence (AI) applications. medRxiv.
Sikora A, Martin GS (2022) Critical Care Pharmacists: Improving Care by Increasing Access to Medication Expertise. Ann Am Thorac Soc. 19(11):1796-1798.
Sikora A, Rafiei A, Rad MG et al. (2023d) Pharmacophenotype identification of intensive care unit medications using unsupervised cluster analysis of the ICURx common data model. Crit Care. 27(1):167.
Sikora A, Zhang T, Murphy DJ et al. (2023e) Machine learning vs. traditional regression analysis for fluid overload prediction in the ICU. medRxiv.
Sikora A, Zhao B, Kong Y et al. (2023f) Machine learning based prediction of prolonged duration of mechanical ventilation incorporating medication data. medRxiv.
Smetana KS, Flannery AH, Gurnani PK et al. (2023) PHarmacist avoidance or reductions in medical costs in CRITically ill adults rounding with one SERVICE compared to two or more services: PHARM-CRIT-SERVICE. Journal of the American College of Clinical Pharmacy. 6(9):1000-1007.
Smith SE, Shelley R, Newsome AS (2021) Medication regimen complexity vs patient acuity for predicting critical care pharmacist interventions. Am J Health Syst Pharm.
Smith SE, Slaughter AA, Butler SA et al. (2021) Examination of critical care pharmacist work activities and burnout. Journal of the American College of Clinical Pharmacy. 4(5):554-569.
Stollings JL, Bloom SL, Wang L et al. (2018) Critical Care Pharmacists and Medication Management in an ICU Recovery Center. The Annals of pharmacotherapy.
Stollings JL, Foss JJ, Ely EW et al. (2015) Pharmacist leadership in ICU quality improvement: coordinating spontaneous awakening and breathing trials. Ann Pharmacother. 49(8):883-91.
Tariq RA, Vashisht R, Sinha A, et al. (2023) Medication Dispensing Errors and Prevention. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available at https://www.ncbi.nlm.nih.gov/books/NBK519065/
National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network; Shapiro NI, Douglas IS et al. (2023) Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 388(6):499-510.
Tversky A, Kahneman D (1974) Judgment under Uncertainty: Heuristics and Biases. Science. 185(4157):1124-1131.
Valentin A, Capuzzo M, Guidet B et al. (2006) Patient safety in intensive care: results from the multinational Sentinel Events Evaluation (SEE) study. Intensive Care Med. 32(10):1591-1598.
van Santvoort HC, Besselink MG, Bakker OJ et al. (2010) A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 362(16):1491-1502.
Vincent JL (2005) Give your patient a fast hug (at least) once a day. Crit Care Med. 33(6):1225-1229.
Webb A, R S., Sikora A (2021) Automated MRC-ICU calculations in the electronic medical record of an academic medical center: Applications and considerations for critical care pharmacist practice. American Journal of Health-System Pharmacy.
Webb AJ, Rowe S, Sikora Newsome A (2022) A descriptive report of the rapid implementation of automated MRC-ICU calculations in the EMR of an academic medical center. Am J Health Syst Pharm.
Webster CS, Taylor S, Weller JM (2021) Cognitive biases in diagnosis and decision making during anaesthesia and intensive care. BJA Educ. (11):420-425.
Wunsch H (2023) The autumn ghost : how the battle against a polio epidemic revolutionized modern medical care. Greystone Books.
Liu Z, Wu Z, Hu M et al. (2023) Pharmacy GPT: The AI Pharmacist.










