ICU Management & Practice, Volume 22 - Issue 4, 2022
Introduction
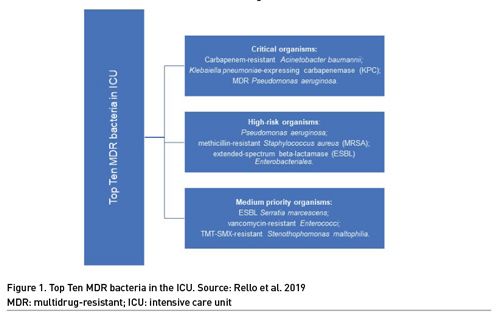
Infections caused by resistant bacteria are associated with higher treatment costs and increased morbidity and mortality, and bacterial resistance represents a major challenge in global health (Antimicrobial Resistance Collaborators, 2022; WHO 2019). Antibiotic stewardship programme (ASP) is one of the interventions aimed at improving the appropriate use of antimicrobials and reducing the incidence of multidrug-resistant (MDR) bacteria (UN 2016). The EPIC II study, a one-day prevalence study, showed that 71% of ICU patients were receiving antibiotics (Vincent et al. 2009). The EPIC III study demonstrated the prevalence of Klebsiella resistant to third generation cephalosporin, Pseudomonas and Acinetobacter reaching rates close to 20% each (Vincent et al. 2020). The main infectious syndromes associated with MDR bacteria are respiratory, bloodstream and intra-abdominal infections (WHO 2021). The Top Ten resistant microorganisms' study (TOTEM) provides a priority pathogen list of the most serious MDR bacteria presenting in the ICU. Carbapenem-resistant (CR) Acinetobacter baumannii, Klebsiella pneumoniae-expressing carbapenemase (KPC), and MDR Pseudomonas aeruginosa were identified as critical organisms (Rello et al. 2019). The other bacteria are listed in Figure 1. Therefore, intensive care units (ICU) are suitable places for the implementation of ASP measures. Similarly, emergency departments (ED) are gateways for community-acquired infections and have unique features of uncertain diagnosis and time pressure that are favourable to misuse of antibiotics (May et al. 2014). This overview aimed to highlight the main aspects related to respiratory infection and sepsis and management in the context of intensive care units and emergency departments.
Antibiotic Stewardship Programme
Antimicrobial stewardship refers to optimised antibiotic prescription to prevent the emergence of antimicrobial resistance (Dyar et al. 2017). Appropriate use of antimicrobials in critical areas as ICUs and EDs means the right drug, at the right time, the right dose, for the right bug, for the right duration (Wunderink et al. 2020). Despite positive evidence with ASP (Atamna-Mawassi et al. 2021; Jover-Sáenz, 2020; Karanika et al. 2016; Lee et al. 2018), in critically ill patients, clinical outcomes remained the primary concern, even with the recognition that antibiotics prescribed for one patient can have ecological negative results. The balance between correct empirical choice and narrow-spectrum antibiotic use may be a challenge, especially in cases of septic shock. Fear of missing causative pathogen or adverse clinical outcomes and aspects concerning patients are important barriers to antibiotic optimisation (Alghamdi et al. 2020; Mathew et al. 2020) (Table 1). Antibiotic optimisation should be a core competency of ICU and ED physicians. Education focused on the appropriate use of antibiotics is key to changing the antimicrobial resistance (AMR) scenario (Wunderink et al. 2020) (Table 2).

Antibiotic Stewardship Programme in Emergency Department
Antibiotics treatment in emergency department (ED) is predominantly empirical and about 30% are inappropriate (Denny et al. 2019; Oomen et al. 2020). The main aspect related to inappropriateness is the indication when there is no need for antibiotics and the broad-spectrum antibiotic regimen (May et al. 2014; Denny et al. 2019). Important details to note when prescribing antibiotic in ED are that the first dose of antibiotic should always be given as a bolus to ensure that the time to peak concentration is not delayed (Vardakas et al. 2018), and should also check that the second dose is administered at the correct interval. Thirty-three per cent of patients with sepsis or septic shock experience a delay in the second dose, and it is more common with frequent dosing intervals (every 8 or 6 hours) as well as hospital admission in the ED (OR 2.67; 95% CI 1.74-4.09) (Leisman et al. 2017). The first infectious syndrome in ED is the respiratory tract infection (McCaig et al. 2006). Thus, we decided to focus on clinical diagnosis and empirical treatment in acute respiratory infections and sepsis in the ED.
Acute Respiratory Tract Infections
Essentially, acute respiratory tract infections (ARTI) are self-limiting viral diseases that do not require antimicrobial treatment. Distinguishing between viral and bacterial infections is essential but may be difficult in some cases. Bacterial pharyngitis can be confirmed by a rapid antigen detection test, throat culture, or both (Petersen et al. 2007). Acute bacterial rhinosinusitis should be considered when there is persistence of symptoms, for more than 10 days without clinical improvement, or worsening of symptoms after few days of evolution (Woodhead et al. 2011). Acute uncomplicated bronchitis is a self-limited inflammation of the large airways, with inflammation lasting more than six weeks. Occasionally, upper respiratory infections and bronchitis may be complicated with pneumonia, particularly in older people after 65 years (Petersen et al. 2007).
Community-acquired pneumonia (CAP) can be caused by viruses and bacteria, and both pathogens can coexist. For younger immunocompetent adults, bacterial pneumonia is difficult without the presence of clinical signs of systemic inflammatory response syndrome (SIRS) (Harris et al. 2006). The American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) (Metlay et al. 2019) guideline published in 2019 and The European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases (ERS/ESCMID) guidelines (Woodhead et al. 2011) have different recommendations for diagnosis and treatment, as described in Table 3. Diagnosis and treatment aspects are described in Figure 2. The optimal duration of therapy for CAP is undefined, but short-term antibiotic (≤6 days) is as effective as long-term (≥7 days) for clinical cure (Tarsali 2018), and discontinuing treatment on fifth day is safe in the absence of fever or other sign of clinical instability for 48 hours (Uganda et al. 2016).


Antibiotic Stewardship Programme in the Intensive Care Unit
Sepsis is diagnosed in about 30% of ICU patients. In a one-day prevalence study in European ICUs, 60% of infections were respiratory tract infections (Vincent et al. 2020). The EU-VAP/CAP study in 27 ICUs in Europe showed that ventilator-associated pneumonia (VAP) is highly prevalent (Koulenti et al. 2017). Considering the importance of sepsis and healthcare-associated infections (Magill et al. 2018), especially hospital-acquired pneumonia (HAP) and VAP, we highlight some useful aspects for antibiotic optimisation in critically ill patients.
Early aetiological diagnosis
Collection of blood samples for blood cultures and other materials is recommended. Blood cultures (BC) require 12-48 hours of incubation to detect the presence of bacteria, and 40% have negative results (Phua et al. 2013); the sensitivity is influenced by fastidious pathogens and reduced on currently antibiotic therapy (Cohen et al. 2015). Automated BC systems can reduce the time needed to detect circulation bacteria, compared to conventional techniques. New molecular diagnostic tests provide results in a few hours, informing about the identification of some types of pathogens as well as genotypic antimicrobial resistance markers. These new tests require less blood volume (10mL) than blood cultures (60mL), which may favour false negatives, since low volume bacteraemia is common. False positives can also occur due to contamination during collection or identification of harmless DNA (DNAemia). Although the results of new genotypic techniques can contribute to traditional methods, the variability of resistance patterns within the same bacterial species makes antimicrobial susceptibility testing (phenotypic evaluation) fundamental in the appropriate choice of antibiotic (Rello and Alonso-Torres 2021). One new technology, the Accelerate PhenoTest BC Kit provides early identification and minimum inhibitory concentration results direct from positive BC, resulting in shorter time to organism identification, shorter time to antimicrobial susceptibility test and to optimal therapy, compared with conventional methods (Bhalodi et al. 2022). This could be a promising technology for the near future. A study comparing conventional bronchoalveolar lavage (BAL) microbiological tests with the rapid diagnostic system by multiplex PCR in suspected VAP found better sensitivity for gram-negative bacteria (90%) than for gram-positive cocci (62%) (Peiffer-Smadja et al. 2020). It is noteworthy that new technologies are expensive, and interpretation and integration of results into patient care require experience in microbiology and knowledge about the limitations of the methods.
Antibiotic de-escalation
Antibiotic de-escalation (ADE) is the replacement of broad-spectrum antimicrobials with a narrower-spectrum agent or stop components of an antimicrobial combination. The ESICM/ESCMID guide to antibiotic de-escalation (Tabah et al. 2020) recommends ADE within 24 hours of definitive culture and antibiogram results. A meta-analysis about empirical antibiotic de-escalation in patients with sepsis and septic shock, identified no significant difference in mortality between the de-escalation group and the group that maintained broad spectrum coverage, suggesting safety of this ADE strategy (Guo et al. 2016). A study of patients with HAP and VAP, identified that de-escalation was associated with fewer antibiotic days (9 vs 11, p<0.001), fewer episodes of Clostridioides difficile infection (2.2% vs 3.8%, p=0.046) and fewer days of hospitalisation (20 vs 22 days, p=0.006); without difference in treatment failure outcome at 30 days (35% ADE vs 33.8% no ADE, p=0.604) (Ilges et al. 2019). However, in clinical practice some aspects disfavour ADE in cases of VAP. Almost 30% of VAP cases have no microorganism identified in respiratory cultures (Rello et al. 2002). Patients without a defined aetiologic agent tend to have higher mortality (Rello et al. 2004). No target makes ADE unlikely; however, there is an opportunity to reassess the indication for antibiotics. In a study with suspected VAP and culture-negative BAL; 66% of cases had alternative aetiology for radiological change (Kollef and Kollef 2005).
Duration of the antibiotic therapy
The optimal duration is the shortest time needed to control the focus of infection. Individualised assessment of the duration of treatment should include host immune status, profile of the pathogen involved, possible complications of infection, pharmacokinetic and pharmacodynamic profile of the antibiotic and clinical stability with treatment. The studies reviewed in the current version of the Surviving Sepsis Campaign (SSC) found no difference in outcomes when comparing short duration (3-8 days in pneumonia; 5-7 days in bacteraemia) and long duration of treatment (7-15 days in pneumonia; 10-14 days in bacteraemia) (Evans et al. 2021). However, a retrospective study with gram-negative BSI, demonstrated a higher risk of treatment failure in short course (7-10 days) compared with long course (>10 days) treatment (hazard ratio 2.60; 95% CI 1.20-5.53, p=0.02) (Nelson et al. 2017). In general, there is a 14-day treatment recommendation for uncomplicated BSI caused by S. aureus (Kimmig et al. 2021; Jung 2018).
The use of biomarkers to guide treatment time has been investigated. In studies with procalcitonin (PTC)-guide algorithm for decision making, it is observed that the PCT cut-off points vary widely, as do the parameters of proportional reduction (Rhee 2016), disfavouring the comparison between the results. Additionally, the cost of PCT is high and there is no proof of cost-effectiveness (Kip et al. 2016). It is highlighted that a single PCT dosage is not enough to exclude bacterial infection (De Santis and Corona 2016). Serum C-reactive protein (CRP) is associated with bacterial load and time-course variations of serum CRP between baseline and 96 hours can be appropriate to assess antibiotic therapy in cases of suspected VAP (Lisboa et al. 2008). Treatment CRP-based protocol was associated with more discontinuation of antibiotic therapy on fifth day compared to the control (35.9% vs 10.6%, OR 4.7, 95% CI 1.9-12, p=0.001) (Borges et al. 2020). These results suggest that CRP may be useful in the evaluation of response to treatment and in the reduction of antibiotic time.
A meta-analysis demonstrated that in VAP caused by gram-negative non-fermentative bacilli, short-term therapy (7-8 days) was associated with a higher risk of recurrence (odds ratio [OR] 2.18, 95% CI 1.14-4.16) (Pugh et al. 2015). In contrast, more recently an open-label, randomised, multicentre trial on Pseudomonas-caused VAP failed to demonstrate noninferiority of the 8-day compared with 15-day treatment; there was greater recurrence of VAP in the 8-day group, although the difference was not statistically significant (Bougle et al. 2022). Gram positive strain identification and "no growth" in BAL cultures are also factors associated with longer treatment time in VAP (Pouliot et al. 2021). It is important to note that in Pseudomonas-positive VAP, persistence of strains in the airway after several days of treatment is frequent, and that lung injury and artificial airways predispose to colonisation (Bodi et al. 2001; Flanagan et al. 2007).
Conclusion
The interventions addressed in this overview are some key steps to ensure appropriate antibiotic treatment in the fight against the spread of bacterial resistance. As take-home messages we can underline: (I) antibiotic optimisation is a core competency of ICU and ED physicians; (II) antibiotic prescribing skills need to be trained; (III) it is essential to know about the pattern of infections and the antibiotic sensitivity profile in each institution, to favour the adequate choice of empirical antibiotic; (IV) re-evaluation regarding the indication of antibiotic in cases of negative microbiological exams; (V) the main obstacles to ADE and reduction in time of treatment is the assistant team's fear of adverse clinical outcomes; and (VI) education about optimisation of antibiotics may be the most effective measure to fight AMR.
Conflict of Interest
None.
References:
Alghamdi S, Atef-Shebl N, Aslanpour Z et al. (2020) Barriers to implementing antimicrobial stewardship programmes in three Saudi hospitals: Evidence from a qualitative study. J Glob Antimicrob Resist. 18:284-90.
Antimicrobial Resistance Collaborators (2019) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 12; 399(10325):629-655.
Atamna-Mawassi H, Huberman-Samuel M, Hershconitz S et al. (2021) nterventions to reduce infections caused by multidrug resistant Enterobacteriaceae (MDR-E): A systematic review and meta-analysis. J Infect. 83(2):156-66.
Bhalodi A, MacVane S, Ford B et al. (2022) Real-World Impact of the Accelerate PhenoTest BC Kit on Patients with Bloodstream Infections in the Improving Outcomes and Antimicrobial Stewardship Study: A Quasiexperimental Multicenter Study. Clin Infect Dis. 75(2):269-277.
Bodi M, Andarnuy C, Olona M et al. (2001) Therapy of ventilator-associated pneumonia: the Tarragona Strategy.Clin Microbiol and Infection. 7(1):32-33.
Borges I, Carneiro R, Bergo R et al. (2020) Duration of antibiotic therapy in critically ill patients: a randomized controlled trial of a clinical and C-reactive protein-based protocol versus an evidence-based best practice strategy without biomarkers. Critical Care. 24:281.
Cohen J, Vincent JL, Adhikari N et al. (2015) Sepsis: a roadmap for future research. Lancet Infect Dis.15:581-614.
Denny K, Gartside J, Alcorn K et al. (2019) Appropriateness of antibiotic prescribing in the Emergency Department. J Antimicrob Chemother. 74(2):515-20.
De Santis V, Corona A (2016) Procalcitonin to guide antibiotic stewardship in intensive care. Lancet Infect Dis.16(8):887-8.
Dyar O, Huttner B, Schouten J et al. (2017) What is antimicrobial stewardship? Clin MicrobiolInfect. 23(11):793-8.
El‑Sokkary R, Uysal S, Erdem H et al. (2021) Profiles of multidrug‑resistant organisms among patients with bacteremia in intensive care units: an international ID‑IRI survey. Eur J Clin Microbiol Infect Dis, 40:2323-2334.
Evans L, Rhodes A, Antonelli M et al. (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 49(11):E1063-143.
Guo Y, Gao W, Yang H et al. (2016) De-escalation of empiric antibiotics in patients with severe sepsis or septic shock: A meta-analysis. Heart Lung. 45(5):454-9.
Harris A, Hicks R, Qaseem A. (2016) Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American college of physicians and the centers for disease control and prevention. Ann Intern Med. 164(6):425-34.
Ilges D, Ritchie D, Krekel T et al. (2019) Assessment of Antibiotic De-escalation by Spectrum Score in Patients With Nosocomial Pneumonia : A Single-Center , Retrospective Cohort Study. Open Forum Infect Dis. 8(11):ofab508.
Jover-Sáenz A, Ramirez-Hidalgo M, Vidal M et al. (2020) Antimicrobial stewardship program at a tertiary care academic medical hospital: Clinical, microbiological and economic impact. A 5-year temporary descriptive study. Infect Prev Pract. 2(2).
Jung N, Rieg S. (2018) Essentials in the management of S. aureus bloodstream infection, Infection. 46(4):441-442.
Karanika S, Paudel S, Grigoras C et al. (2016) Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrobial Agents and Chemotherapy. 60:4840–52.
Kimmig A, Hagel S, Weis S et al. (2021) Management of Staphylococcus aureus Bloodstream Infections Front.Med. 7:616524.
Kip M, Koffijberg H, Ijzerman M et al. (2016) Procalcitonin to guide antibiotic stewardship in intensive care. Lancet Infect Dis. 16(8):888.
Kollef M, Kollef K (2005) Antibiotic Utilization and Outcomes for Patients With Clinically Suspected Ventilator-Associated Pneumonia and Negative Quantitative BAL Culture Results. Chest. 128:2706–2713.
Koulenti D, Tsigou E, Rello J (2017) Nosocomial pneumonia in 27 ICUs in Europe : perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 36:1999-2006.
Lee C, Cowling B, Feng S et al. (2018) Impact of antibiotic stewardship programmes in Asia: A systematic review and meta-analysis. J Antimicrob Chemother. 73(4):844-51.
Leisman D, Huang V, Zhou Q et al. (2017) Delayed second dose antibiotics for patients admitted from the emergency department with sepsis: Prevalence, risk factors, and outcomes. Crit Care Med. 45(6):956-65.
Lisboa T, Seligman R, Diaz Emili et al. (2008) C-reactive protein correlates with bacterial load and appropriate antibiotic therapy in suspected ventilator-associated pneumonia. Crit Care Med. 36:166-171.
Magill S, O´Leary E, Janelle S et al. (2018) Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N Engl J Med. 379(18):1732-44.
Mathew P, Ranjalkar J, Chandy S (2020) Challenges in Antimicrobial Stewardship Implementation; An exploratory study. Front. Public Health. 8:493904.
May L, Gudger G, Armstrong P et al. (2014) Multisite Exploration of Clinical Decision-Making for Antibiotic Use by Emergency Medicine Providers Using Quantitative and Qualitative Methods. Infect Control Hosp Epidemiol. 35(9):1114-25.
McCaig LF, Nawar EW (2006) National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary, Adv Data. 372:1-29.
Metlay J, Waterer G, Long A et al. (2019) Diagnosis and treatment of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 200(7):E45–67.
Nelson A, Justo J, Bookstaver P et al. (2017) Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 45(5):613-20.
Oomen P, Dutilh J, Logtenberg S et al. (2020) Appropriate Antibiotic Prescribing in the Emergency Department A Cross-sectional Study. Infect Dis Clin Pract. 30(1).
Peiffer-Smadja N, Bouadma L, Mathy V et al. (2020) Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Critical Care. 24:366;1-10.
Petersen I, Johnson A, Islam a et al. (2007) Protective effect of antibiotics against serious complications of common respiratory tract infections: Retrospective cohort study with the UK General Practice Research Database. Br Med J. 335(7627):982-4.
Phua J, Ngerng W, See K et al. (2013) Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 17(5):R202.
Pouliot J, Dortch M, Givens G et al. (2021) Factors Associated With Prolonged Antibiotic Use in the Setting of Suspected Pneumonia and Negative Bronchoalveolar Lavage Cultures. Hospital Pharmacy. 56(5) 444-450.
Pugh R, Grant C, Cooke R et al. (2015) Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev.
Ramirez-Estrada, Borgatta B, Rello J. (2016) Pseudomonas aeruginosa ventilator-associated pneumonia management. Infection and Drug Resistance. 9:7-18.
Rello J, Ollendorf D, Oster G et al. (2002) Epidemiology and Outcomes of Ventilator-Associated Pneumonia in a Large US Database. Chest. 122:2115-2121.
Rello J, Vidaur L, Sandiumenge A et al. (2004) De-escalation therapy in ventilator-associated pneumonia. Crit Care Med. 32(11):2183-2190.
Rello J, Eshwara V, Lagunes L et al. (2019) A global priority list of the Top Ten resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur J Clin Microbiol Infect Dis. 38:319-323.
Rello J, Alonso-Tarres C (2021) Emerging Technologies for Microbiologic Diagnosis of Sepsis: The Rapid Determination of Resistance to Antimicrobial Agents Should Be the Key. Clinical Infectious Diseases. 73(7):1173-5.
Rhee C. (2016) Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis. 1-10.
Rioux J, Edwards J, Bresee L et al. (2017) Nasal-Swab results for methicillin-resistant staphylococcus aureus and associated infections. Can J Hosp Pharm. 70(2):107-12.
Tabah A, Basseti M, Kollef M et al. (2020) Antimicrobial de ‑ escalation in critically ill patients : a position statement from a task force of the European Society of Intensive Care Medicine ( ESICM ) and European Society of Clinical Microbiology and Infectious Diseases ( ESCMID ) Critically Ill Patients Study Group ( ESGCIP ). Intensive Care Med. 46(2):245-65.
Tarsali G, Mylonakis E (2018) Systematic review and meta-analysis of the efficacy of short-course antibiotic treatments for community-acquired pneumonia in adults. Antimicrob Agents Chemother. 62(9):e006:1-13.
United Nations, General Assembly (2016) Resolution adopted by the General Assembly on 5 October 2016. Political declaration of the high-level meeting of the General Assembly on antimicrobial resistance. Available from https://press.un.org/en/2016/ga11835.doc.htm
Vardakas K, Voulgaris G, Maliaros A et al. (2018) Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 18(1):108-20.
Vincent JL, Rello J, Marshall J et al. (2009) International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 302(21):2323-9.
Vincent JL, Sakr Y, Singer M et al. (2020) Prevalence and Outcomes of Infection among Patients in Intensive Care Units in 2017. JAMA. 323(15):1478-87.
World Health Organization (2019) New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available from https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis
World Health Organization (2021) Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021. Geneva;167.
Woodhead M, Biasi F, Ewig S et al. (2011) Guidelines for the management of adult lower respiratory tract infections. Clin Microbiol Infect. 17(Suppl.6):E1-59.
Wunderink R, Srinivasan A, Barie P et al. (2020) Antibiotic Stewardship in the Intensive Care Unit An Official American Thoracic Society. Ann Am Thorac Soc. 17(5):531-40.









