ICU Management & Practice, ICU Volume 12 - Issue 1 - Spring 2012
Acute kidney injury (AKI) is a hot topic in the medical arena, with prominent research and discussion ongoing, prompting amendments to recommended practice. The Acute Kidney Injury Network (AKIN) and Acute Dialysis Quality Initiative (ADQI) and AKI section of the European Society Intensive Care Medicine (ESICM) continue to work on counsel for clinical practice and research, while new guidelines from Kidney Disease: Improving Global Outcomes (KDIGO) are published recently (Kidney International, March 2012). For this issue of ICU Management, we asked three influential figures in the field, John Kellum, Professor of Critical Care Medicine at the University of Pittsburgh, USA; Claudio Ronco, Professor of Nephrology Dialysis & Transplantation, based at San Bortolo Hospital, Vicenza, Italy; and Michael Joannidis, Associate Professor of Intensive Care at the Medical University Innsbruck, Austria to provide their opinions on developments within the field.
What Does the Emerging Consensus Recommend for Early Identification and Definition of AKI?
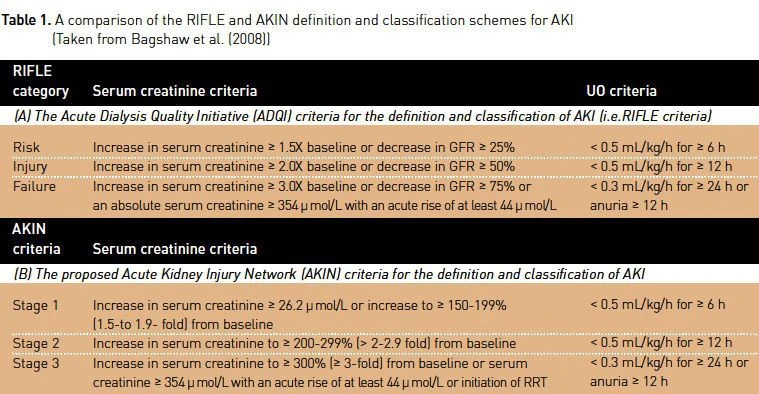
John Kellum: It is now clear that patients may present with evidence of AKI or they may acquire it after hospitalisation. It is also clear that patients with and without preexisting chronic kidney disease (CKD) are at risk. For these reasons, the criteria for AKI must allow for either a change in function from baseline, or a small ongoing change under observation. The AKIN modification to the original RIFLE criteria (see Table 1) allows for either scenario and thus represents the best way to define AKI. It is not sufficient to simply use the original RIFLE criterion for creatinine because patients with elevated baseline creatinine would be too often missed. Furthermore, it is unacceptable to require ongoing increases in creatinine to define AKI, since patients with community acquired AKI may present their worst creatinine. The just published KDIGO has further clarified these points.
Another significant issue that has been highlighted is that serum creatinine is not a very good early marker of AKI and better markers are needed. Changes in urine flow occur earlier than creatinine but they lack sensitivity and specificity. New biomarkers like NGAL, KIM-1, IL-18 and FABP are being validated for the use in early identification of AKI and have the potential to change clinical practice.
How Important is the Early Identification of Acute Kidney Injury?
John Kellum: It is very important. Like most diseases, AKI is harder to treat when it is detected late, resulting in lower chances of recovery. In the early stages of AKI, kidney cells are usually still viable, and the potential exists to mitigate disease progression with effective therapy.
Is the Prevention of AKI Possible?
John Kellum: Yes, without a doubt. Many cases of AKI occur in already hospitalised patients and appear to be the result of multiple insults, including nephrotoxic drug exposure, radio contrast, and sepsis. Careful attention to these exposures in high risk patients will reduce the incidence of AKI. However, most cases of AKI are caused by conditions that are not modifiable, and often in these instances patients present an already advanced disease. For these patients, the focus of medical care should be on reducing continued injury and facilitating renal recovery.
What is the Best Intervention to Prevent Acute Kidney Injury in the Critically Ill?
John Kellum: There is no single method for prevention of AKI. Avoidance of unnecessary nephrotoxins, and rapid and effective treatment of infection are likely to be the most efficient preventative measures, while ensuring adequate intravascular volume status and avoiding volume overload are also paramount. Effective volume management is not merely giving fluid for low urine output and then attempting to take it away with diuretics. It is important to understand that once the kidney is injured, urine output is no longer a useful gauge of fluid status; therefore, the physician must seek other methods to determine the right amount of fluid to be given. This may include functional haemodynamic monitoring such as pulse-pressure variation.
Michael Joannidis: In this context it should be added that the paradigm of fluid management is currently changing and first data have been presented indicating that under the condition of sufficient haemodynamic stability, keeping patients on the 'dryer' side may protect renal function even more effectively than unguided fluid loading.
In Cases of Liver Failure, What can We do to Prevent Acute Renal Failure from Developing?
Michael Joannidis: Preservation of renal function in chronic liver cirrhosis consists of applying hyperoncotic albumin in combination with vasopressors (e.g. terlipressin). Furthermore, early antibiotic treatment is recommended for any signs of infection as well as episodes of gastrointestinal bleeding
What can We do to Prevent Acute Renal Failure from Developing in Patients with Lung Injury?
Michael Joannidis: Currently, no specific interventions in addition to the general measures described above by John Kellum and myself have proven efficient in the setting of lung injury. Fluid restriction may help both the lung and the kidney in this setting as long as adequate renal perfusion is granted. Furthermore, limiting additional inflammatory injury from mechanical ventilation by applying non invasive ventilation or reducing strain through low tidal volumes if intubation cannot be avoided results in lower circulation cytokine levels and reduced rates of AKI.
Have Any Preventative Developments Been Made in Cases of Cardiac Surgery?
Michael Joannidis: The use of vasodilators (e.g. fenoldopam) or inodilator (e.g. levosimendan) has been proposed by some studies to be a promising approach for preventing AKI in these instances, although the strength of data available is still considered insufficient. What about in cases of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS)?
Michael Joannidis: Prevention of IAH is of high relevance, with effective measures consisting of limiting positive fluid balance and ensuring gut function by applying early enteral nutrition (EN). In the case of ACS surgical intervention may be unavoidable.
Should We Use Crystalloids or Colloids for Fluid Resuscitation?
Michael Joannidis: Currently isotonic crystalloids are considered first choice for fluid resuscitation which is also reflected by recent recommendations of a task force of ESICM. This preference results from several recent publications indicating possible renal impairment by synthetic colloids, especially starches. The role of hyperoncotic albumin in critically ill patients is currently under investigation by two large RCTs.
What Role do Diuretics Play in the Management of Acute Kidney Injury?
John Kellum: Diuretics should only be used to treat or avoid volume overload; they are not treatments for AKI per se and offer no benefit to the kidney. In fact, data-form observational studies suggest harmful risks exist in the use of diuretics, causing some clinicians to favour using extracorporeal therapy for managing fluid and solute in patients with AKI. Still, diuretics will remain an irreplaceable tool for management of volume in critically ill patients with and without AKI.
What is the Role of Renal Replacement Therapy (RRT) in the Management of Acute Kidney Injury?
John Kellum: Emerging evidence suggests that early RRT leads to better outcomes for patients with severe AKI. The difficulty lies in deciding which patients will ultimately require RRT and which will experience rapid spontaneous recovery. AKI biomarkers may play a role in this process. Another question is that of modality. CRRT is gentle and unlikely to result in further insult to a injured kidney, whereas intermittent haemodialysis has the potential to cause further damage. Based on current evidence, we recommend initiating RRT prior to complications such as volume overload and hyperkalaemia. In patients at risk for haemodynamic instability or intracranial hypertension we recommend using CRRT, while we also recommend CRRT when intermittent haemodialysis is insufficient to obtain adequate fluid and solute management.
How Can the Best Starting Time for RRT be Determined?
Claudio Ronco: The issue of the appropriate start of extracorporeal techniques in the critically ill patient has long been debated by experts, consensus, organisations and evidence panels. The modern view is that an early application of extracorporeal techniques may provide benefits that prevail over the potential draw backs of futile exposure of a patient to unnecessary blood/artificial material contact. Benefits include the control of fluid balance, avoidance of severe derangements and prevention rather than correction of biochemical and clinical disorders.
Recently, techniques and related equipment have become so simple and user-friendly that no application of an extracorporeal therapy, even in critically ill patients, is seen as a risk, rather they are viewed as a promising opportunity. The classic indications represented by oliguria, azotaemia and other uremic complications are considered as extreme consequences of an inappropriate strategy of prevention and management. Furthermore, extracorporeal techniques are considered to be a method of support rather than a replacement of the failing organ. For this reason, indications for the initiation of treatment have changed and have become softer, justifying an early start.
Early versus late is a terminology that may become complex and misleading unless we define exactly a reference parameter, since different meanings are construed from these terms depending on creatinine, urine output or other biochemical parameters used. We advocate that the introduction of RIFLE/AKIN AKI stages has brought about a new dimension in response to this problem. We now have fixed criteria to define AKI and to stage it. A new option has become available thanks to early AKI biomarkers such as NGAL, Cystatin C and KIM-1, and the diagnosis of AKI may be possible today even in the absence of oliguria or a rise in serum creatinine. The biomarker positive patient without creatinine or urine output abnormalities has been shown to have a worse prognosis and more severe outcome; thus, the entire concept of early should be revisited in light of these recent acquisitions.
Finally, it should be noted that if an early start of RRT is applied, we might end up treating patients who would have improved anyway, or in which the treatment is futile and unecessarily costly. Clinical trials so far conducted seem to rule against this hypothesis, although a solid body of evidence is still missing. Our clinical experience conveys that every patient is a self standing case and we should carefully evaluate the patient with a multidisciplinary approach to decide on the best start time for RRT.
Which Types of Patients Would Best Benefit From Continuous Renal Replacement Therapy (CRRT) and Which From Intermittent Haemodialysis (IHD)?
Claudio Ronco: First of all we must say that CRRT and IHD are not opposite therapies but rather two schedules of the same treatment in a continuum that goes from short intermittent sessions to continuous 24/7 treatments. For this reason, one patient may shift from one schedule to another during his/her hospital stay.
In general, we may say that due to the characteristics of each schedule, patients with haemodynamic instability, at risk for brain oedema and/or presenting signs and symptoms of severe sepsis and septic shock, are most likely to find benefits from continuous therapies. On the other hand, patients that are haemodynamically stable or that are moving from a severe illness to a less severe clinical condition with improved haemodynamics may benefit from a discontinuation of CRRT, receiving occasional sessions of isolated haemodialysis/haemofiltration.
Several debates and controversies exist regarding CRRT versus IHD, as there is no compromise or intermediate approach. However, studies comparing continuous and intermittent therapies have failed to demonstrate the superiority of one schedule against the other. Today critically ill patients admitted to ICU are most likely to receive CRRT whilst patients with isolated AKI, outside ICU, are most likely to receive a daily or thrice weekly schedule of intermittent IHD.
What Changes do You Foresee in the Treatment of AKI in the Coming Decade?
John Kellum: The near future is likely to see greater use of AKI biomarkers for risk assessment. Also, increased recognition of AKI, bolstered in large part by the many consensus documents being published, will result in better care and increased research. The results will hopefully be the development of novel therapies designed to attenuate renal injury and/or facilitate renal recovery. Finally, we anticipate additional studies to settle the issue of when to initiate RRT.
Which Emerging Technologies in the Field Excite You the Most?
John Kellum: Therapies targeted at blocking inflammation in the kidney rather than manipulating the circulation interest me a lot. Also fascinating is the emergence of better forms of blood purification with the ability to remove molecules that perpetuate damage in the kidney.
Claudio Ronco: What excites me the most is Multiple Organ Support Therapy (MOST). New CRRT platforms have the capability to process blood in a series of extracorporeal circuits and devices that allow for renal, liver, cardiac and pulmonary support. The capacity to simultaneously remove uremic waste products and CO2, combining renal and pulmonary support, is opening new interesting avenues in the care of critically ill patients. By adding adsorption devices capable of removing endotoxin and septic mediators, one can really achieve a universal treatment for organ support in critical illness and sepsis. Studies should be designed to test the effects of such new therapies on solid endpoints in well-designed clinical trials.

References:
Bagshaw R et al. (2008). A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant, 23:1569–1574.
Kidney Disease: Improving Global Outcomes (KDIGO) (2012). KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International