Fibrinogen limits coagulopathy and massive bleeding, has less transfusion requirements and thereby decreases the risk of multi-organ failure in trauma patients.
What stops the bleeding?
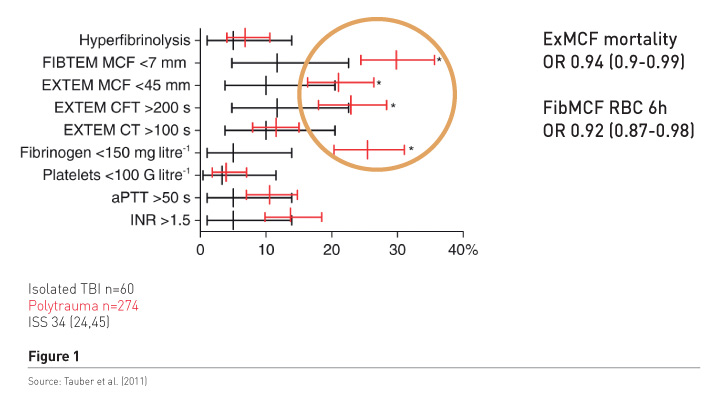
Haemostatic therapy aims to stop the bleeding, but is it a concentration of coagulation factors, mainly assessed by international normalised ratio (INR) readings that works, or is it fibrinogen/fibrin, which are the precondition for stable clot formation? We conducted a study in patients with polytrauma and those with isolated brain injury (Tauber et al. 2011) to find out what the most predominant pathology was (Figure 1). The red bars refer to polytrauma patients. There is significant increase in the frequency of low fibrinogen, low fibrinpolymerization and consequently low clot firmness, in 20-30%, while a significant and prolonged INR of about 1.5 was found in only about 14%. Clot firmness and fibrin polymerization were independently associated with mortality and also with blood loss as measured by early transfusion requirements. In addition patients had tremendously increased molecular markers of thrombin generation, regardless of the INR readings. It’s not the main interest to increase it more by substituting plasma, because very huge thrombin levels do not benefit trauma patients. It may cause endothelial injury and also activate other receptors and inflammation and so on.
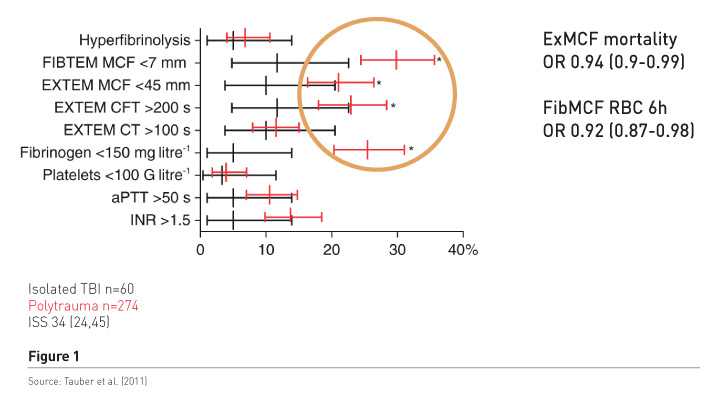
Further results confirm these findings, e.g. in a study that included more than 4,000 patients, with injury severity scores (ISS) considerably lower than in our patient population, fibrinogen deficiency occurred frequently and fibrinogen levels < 1.5g/L were associated with increased mortality (McQuilten et al. 2017). Hagemo et al.’s study investigating 1,133 patients in a multicentre trial, found that increased mortality was associated with fibrinogen levels < 2.29 g/L, which is barely below normal (Hagemo et al. 2014). The INR was not independently associated with mortality.
Coagulation factor substitutes
Plasma
Plasma refers to 6-8% protein solution and 92-94% water. It was introduced in clinical practice mainly for volume substitution but later to treat coagulation disorders. It contains all procoagulants and also anticoagulants. It’s easy to use, is considered safe regarding thrombosis, and relatively low-cost. However, plasma transfusion is time-consuming and requires planning.
The concentration of coagulation factors and especially fibrinogen are rather low in plasma and vary depending on the individual donor and the type of processing. Plasma efficacy can be questioned and partial requirements correction is not possible. It may also induce transfusion-associated circulatory overload (TACO), transfusion-associated lung injury (TRALI), transfusion-associated immunomodulation (TRIM), multi-organ failure (MOF), immunosuppression, lung injury.
Fibrinogen concentrate
There are several concentrates on the market:
1. Fibrinogen concentrate
2. FXIII concentrate
3. PCC (FII, VII, IX, X)
4. vWF concentrate
5. rVIIa, PCCa
6. FVIII, IX, X, XI concentrate
No factor V concentrate is available.
Concentrates are immediately ready to use, contain defined and high concentration of the factor, no volume expansion is needed, so there will be an effective rise in concentration, making targeted therapy possible. There will be no TACO, TRALI or TRIM and the concentrates are virus-inactivated.
The main problem is cost; they are more expensive than plasma. Some may be concerned about the risk of thromboembolism, and this may occur if thrombin formation is increased by use of PCC and activated PCC and rFVIIa. It is not a problem with fibrinogen, which is also called antithrombin I, as fibrinogen and fibrin are able to capture free-flowing thrombin, and thrombin is the one that initiates thrombosis. Authors of reviews and meta-analyses also criticise the fact that currently there are only a few high-quality studies in trauma patients showing a benefit with coagulation factor concentrates.
The European guideline (Rossaint et al. 2016) recommends the use of standard coagulation tests and/or viscoelastic tests (level of evidence 1C). Viscoelastic testing gives a timely and more comprehensive picture. The guideline recommends use of plasma together with RBC at least 1:2 or use of fibrinogen concentrate with RBC and PCC and factor XIII in selected cases.
Evidence for fibrinogen concentrate
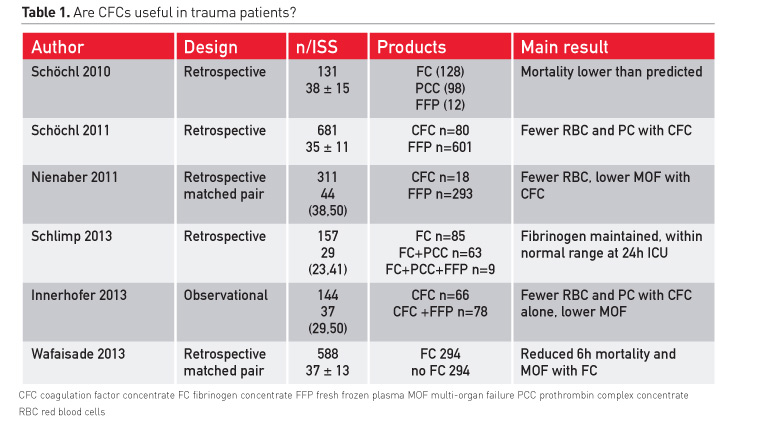
The most frequently cited studies using coagulation factor concentrates in trauma patients are summarised in Table 1. All show promising results: lower mortality than predicted, lower transfusion requirements, and lower multi-organ failure. Fibrinogen was maintained within a normal range even if much fibrinogen had been administered. There were fewer transfusions of red blood cells (RBCs) and platelets. In the study by Wafaisade and colleagues (2013), early mortality was also reduced. These patients received fibrinogen and also had massive transfusions of plasma.
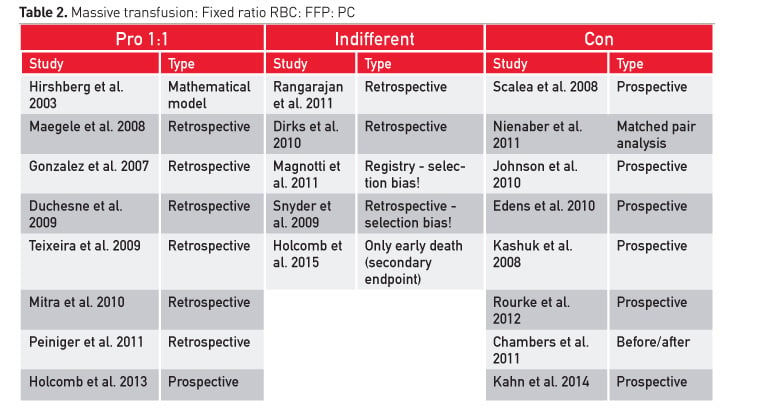
Meta-analyses on plasma efficacy in bleeding patients have concluded that there is no clear benefit for blood loss, transfusion and mortality (Stanworth et al. 2004; Casbard et al. 2004; Yang et al. 2012; Kozek-Langenecker et al. 2011; Desborough et al. 2015). However, there are several reports in trauma patients showing improved survival with early aggressive transfusion without any blood measurements. Administration of 1:1 ratios is recommended, but there are studies that found that mortality did not change and coagulopathy was not corrected (Table 2).
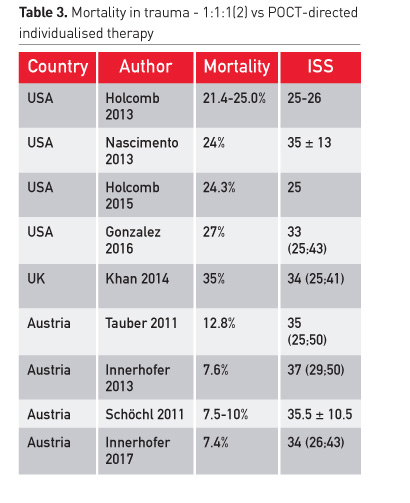
Before massive transfusion of plasma, reported mortality in many centres was about 50 percent. Now the rate is between 21% and 35% (Table 3). This mortality is considerably higher than studies using a targeted correction of coagulopathy and using coagulation factor concentrates, especially fibrinogen concentrate.
RETIC trial comparing plasma and coagulation factor concentrates
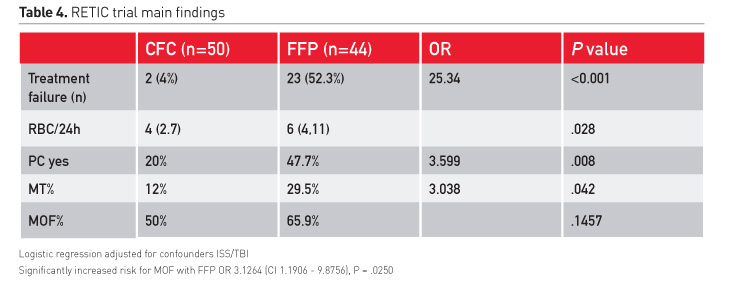
Our study group conducted the first randomised controlled trial comparing the effect of a plasma-based strategy to the use of coagulation factor concentrate in severe trauma (Innerhofer 2017). The study was terminated early following interim analysis after inclusion of 100 patients, because predefined stopping was met, showing disadvantages with use of plasma. Correction of coagulopathy was feasible in 96% of patients in the CFC group; only 2 patients had treatment failure and also received plasma (Table 4).
In the plasma group more than 50% of patients had no stop of bleeding and no correction of coagulation. These patients received additionally fibrinogen concentrate, some also PCC and factor XIII. Transfusion requirements were increased and patients received more frequently platelet concentrates. Importantly, the rate of massive transfusion at comparable ISS was increased tremendously.
Our primary endpoint was difference in MOF. However, to answer this question, we would have needed to include at least 200 patients. Therefore the difference of 16% between groups with a higher rate of MOF in the plasma group was not significant. However, ISS and brain injury are confounders, which should be considered when analysing the likelihood of MOF. These confounders were used for stratification and considered in regression analysis. Results showed a significant increased risk of MOF with plasma (OR 3·13 [1·19–8·88], p=0·025) even in this limited population of 94 patients.
What stops the bleeding–the concentration or the clot?
In the RETIC study the blue boxes refer to the CFC group that mainly received fibrinogen concentrate. The yellow boxes refer to the plasma group (Figure 2). Prothrombin is indexed as a percentage of normal, and at baseline they are comparable. After administration of plasma this improved, but decreased further in the CFC group. The patients in the plasma group received more RBC and platelets concentrates and, despite this, had a dramatic drop in platelet count. Also the haemoglobin levels were lower than in the CFC group that received fewer RBCS and platelets. Therefore improved INR or prothrombin time does not limit blood loss. What about fibrinogen and clot firmness? Fibrinogen increased to its normal levels immediately with the factor-based concept, but changed little and marginally and remained below normal in the plasma group. Consequently clot strength improved rapidly with CFC but remained unchanged or decreased in the plasma group.
Conclusion/ Key points
- Early and effective fibrinogen supplementation is really important to limit blood loss and minimise risk of MOF
- Fibrinogen improves clot strength and also exhibits a platelet-saving effect.This is very important because platelets are one of the transfusion components that are sometimes dangerous and have many side effects
- Fibrinogen limits coagulopathy and massive bleeding and has less transfusion requirements especially massive transfusion and thereby it also decreases the risk of MOF
- An effective rise of fibrinogen concentration is not feasible with plasma
- The lower, better INR after plasma does not reduce the bleeding, therefore we should not focus on the INR
- Fibrinogen is of interest, should be monitored and should be supplemented early
Abbreviations
ISS injury severity score
MOF multi-organ failure
TACO transfusion-associated circulatory overload
TRALI transfusion-associated lung injury
TRIM transfusion-associated immunomodulation
<!--
/* Font Definitions */
@font-face
{font-family:Cambria;
panose-1:2 4 5 3 5 4 6 3 2 4;
mso-font-alt:"Times New Roman";
mso-font-charset:77;
mso-generic-font-family:roman;
mso-font-format:other;
mso-font-pitch:auto;
mso-font-signature:3 0 0 0 1 0;}
/* Style Definitions */
p.MsoNormal, li.MsoNormal, div.MsoNormal
{mso-style-parent:"";
margin:0cm;
margin-bottom:.0001pt;
mso-pagination:widow-orphan;
font-size:12.0pt;
font-family:"Times New Roman";
mso-ascii-font-family:Cambria;
mso-ascii-theme-font:minor-latin;
mso-fareast-font-family:Cambria;
mso-fareast-theme-font:minor-latin;
mso-hansi-font-family:Cambria;
mso-hansi-theme-font:minor-latin;
mso-bidi-font-family:"Times New Roman";
mso-bidi-theme-font:minor-bidi;}
@page Section1
{size:612.0pt 792.0pt;
margin:72.0pt 90.0pt 72.0pt 90.0pt;
mso-header-margin:36.0pt;
mso-footer-margin:36.0pt;
mso-paper-source:0;}
div.Section1
{page:Section1;}
-->
Casbard AC, Williamson LM, Murphy MF et al. (2004) The role
of prophylactic fresh frozen plasma in decreasing blood loss and correcting
coagulopathy in cardiac surgery. A systematic review. Anaesthesia, 59(6):
550-8.
Chambers LA, Chow SJ, Shaffer LE (2011) Frequency and
characteristics of coagulopathy in trauma patients treated with a low- or
high-plasma-content massive transfusion protocol. Am J Clin Pathol, 136(3):
364-70.
Davenport R, Curry N, Manson J et al. (2011) Hemostatic
effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J
Trauma, 70(1): 90-5.
Desborough M, Sandu R, Brunskill SJ et al. (2015) Fresh
frozen plasma for cardiovascular surgery. Cochrane Database Syst Rev, (7): CD007614.
Dirks J,
Jørgensen H, Jensen CH et al. (2010) Blood product ratio in acute
traumatic coagulopathy--effect on mortality in a Scandinavian level 1 trauma
centre. Scand J Trauma Resusc Emerg Med, 18: 65.
Duchesne JC, Islam TM, Stuke L et al. (2009) J Trauma,
67(1): 33-7. Hemostatic resuscitation during surgery improves survival in
patients with traumatic-induced coagulopathy.
Edens JW, Chung
KK, Pamplin JC et al. (2010) Predictors of early acute lung injury at a
combat support hospital: a prospective observational study. J Trauma, 69 Suppl
1: S81-6.
Gonzalez EA, Moore FA, Holcomb JB et al. (2007) Fresh frozen
plasma should be given earlier to patients requiring massive transfusion. J
Trauma, 62(1): 112-9.
Gonzalez E, Moore EE, Moore HB et al. (2016) 13.
Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a
pragmatic randomized clinical trial comparing a viscoelastic assay to conventional
coagulation assays. Ann Surg, 263(6): 1051-9.
Hagemo JS, Stanworth S, Juffermans NP et al. (2014)
Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a
multicentre observational study. Crit Care, 18(2): R52.
Hirshberg A, Dugas M, Banez EI et al. (2003) Minimizing
dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J
Trauma, 54(3): 454-63.
Holcomb JB, del Junco DJ, Fox EE et al.; PROMMTT Study Group
(2013) The prospective, observational, multicenter, major trauma transfusion
(PROMMTT) study: comparative effectiveness of a time-varying treatment with
competing risks. JAMA Surg, 148(2): 127-36.
Holcomb JB, Tilley BC, Baraniuk S et al. (2015) Transfusion
of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and
mortality in patients with severe trauma: the PROPPR randomized clinical trial.
JAMA, 313(5): 471-82.
Innerhofer P, Westermann I, Tauber H et al. (2013) The
exclusive use of coagulation factor concentrates enables reversal of
coagulopathy and decreases transfusion rates in patients with major blunt
trauma. Injury, 44(2): 209-16.
Innerhofer P, Fries D, Mittermayr M et al. (2017) Reversal
of trauma-induced coagulopathy using first-line coagulation factor concentrates
or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label,
randomised trial. Lancet Haematol, 4(6): e258-e271.
Johnson JL, Moore EE, Kashuk JL et al. (2010) Effect of
blood products transfusion on the development of postinjury multiple organ
failure. Arch Surg, 145(10): 973-7.
Kashuk JL, Moore EE, Johnson JL et al. (2008) Postinjury
life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood
cells the answer? J Trauma, 65(2): 261-70.
Khan S, Brohi K, Chana M et al; International Trauma Research
Network (INTRN) (2014) Hemostatic resuscitation is neither hemostatic nor
resuscitative in trauma hemorrhage. J Trauma Acute Care Surg, 76(3): 561-7.
Kozek-Langenecker S, Sørensen B, Hess JR et al. (2011)
Clinical effectiveness of fresh frozen plasma compared with fibrinogen
concentrate: a systematic review. Crit Care, 15(5): R239.
Maegele M, Lefering R, Paffrath T et al.; Working Group on
Polytrauma of the German Society of Trauma Surgery (DGU) (2008) Red-blood-cell
to plasma ratios transfused during massive transfusion are associated with
mortality in severe multiple injury: a retrospective analysis from the Trauma
Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang, 95(2): 112-9.
Magnotti LJ,
Zarzaur BL, Fischer PE et al. (2011) Improved survival after hemostatic
resuscitation: does the emperor have no clothes? J Trauma, 70(1): 97-102.
McQuilten ZK, Wood EM, Bailey M et al. (2017) Fibrinogen is
an independent predictor of mortality in major trauma patients: A five-year
statewide cohort study. Injury, 48(5): 1074-81.
Mitra B, Mori A, Cameron PA et al. (2010) Fresh frozen
plasma (FFP) use during massive blood transfusion in trauma resuscitation.
Injury, 41(1): 35-9.
Nascimento B, Callum J, Tien H, et al. (2013) Effect of a
fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided
transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ, 185(12): E583-9.
Nienaber U,
Innerhofer P, Westermann I et al. (2011) The impact of fresh frozen
plasma vs coagulation factor concentrates on morbidity and mortality in
trauma-associated haemorrhage and massive transfusion. Injury, 42(7): 697-701.
Peiniger S, Nienaber U, Lefering R et al.; Trauma Registry
of the Deutsche Gesellschaft für Unfallchirurgie (2011) Balanced massive
transfusion ratios in multiple injury patients with traumatic brain injury.
Crit Care, 15(1): R68.
Rangarajan K, Subramanian A, Pandey RM (2011) Determinants
of mortality in trauma patients following massive blood transfusion. J Emerg
Trauma Shock, 4(1): 58-63.
Rossaint R, Bouillon B, Cerny V et al. (2016) The European
guideline on management of major bleeding and coagulopathy following trauma:
fourth edition. Crit Care, 20: 100.
Rourke C, Curry N, Khan S et al. (2012) Fibrinogen levels
during trauma hemorrhage, response to replacement therapy, and association with
patient outcomes. J Thromb Haemost, 10(7): 1342-51.
Scalea TM, Bochicchio KM, Lumpkins K et al. (2008) Early
aggressive use of fresh frozen plasma does not improve outcome in critically
injured trauma patients. Ann Surg,
248(4): 578-84.
Schlimp CJ,
Voelckel W, Inaba K et al. (2013) Impact of fibrinogen concentrate alone
or with prothrombin complex concentrate (+/- fresh frozen plasma) on plasma
fibrinogen level and fibrin-based clot strength (FIBTEM) in major trauma: a
retrospective study. Scand J Trauma
Resusc Emerg Med, 21: 74.
Schöchl H,
Nienaber U, Hofer G et al. (2010) Goal-directed coagulation management
of major trauma patients using thromboelastometry (ROTEM)-guided administration
of fibrinogen concentrate and prothrombin complex concentrate. Crit Care, 14(2): R55.
Schöchl H,
Nienaber U, Maegele M et al. (2011) Transfusion in trauma:
thromboelastometry-guided coagulation factor concentrate-based therapy versus
standard fresh frozen plasma-based therapy. Crit Care, 15(2): R83.
Snyder CW, Weinberg JA, McGwin G Jr et al. (2009) The
relationship of blood product ratio to mortality: survival benefit or survival
bias? J Trauma, 66(2): 358-62.
Stanworth SJ, Brunskill SJ, Hyde CJ et al. (2004) Is fresh
frozen plasma clinically effective? A systematic review of randomized
controlled trials. Br J Haematol, 126(1): 139-52.
Tauber H,
Innerhofer P, Breitkopf R et al. (2011) Prevalence and impact of
abnormal ROTEM(R) assays in severe blunt trauma: results of the 'Diagnosis and
Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study'. Br J Anaesth,
107(3): 378-87.
Teixeira PG, Inaba K, Shulman I et al. (2009) Impact of
plasma transfusion in massively transfused trauma patients. J Trauma, 66(3):
693-7.
Wafaisade A, Lefering R, Maegele M et al.; Trauma Registry
of DGU (2013)
Administration of fibrinogen concentrate in exsanguinating
trauma patients is associated with improved survival at 6 hours but not at
discharge. J Trauma Acute Care Surg, 74(2):387-93.
Yang L, Stanworth S, Hopewell S et al. (2012) Is
fresh-frozen plasma clinically effective? An update of a systematic review of
randomized controlled trials. Transfusion, 52(8):1673-86.