A case report published in JAMIA Open highlights the insufficient characterization of interoperability in pathology data exchange, despite the emphasis on health information exchange (HIE). It evaluates the fidelity of laboratory data shared between two healthcare institutions through a commonly used HIE construct. The lack of access to relevant clinical data from multiple providers can potentially harm patients. Successful laboratory data interoperability necessitates accurate data exchange and recognition of comparable test types and results. Efforts such as the Systemic Harmonisation and Interoperability Enhancement for Laboratory Data (SHIELD) aim to enhance patient care and prevent safety issues. Semantic standards are crucial for achieving interoperability, including LOINC terms for questions, SNOMED CT codes for qualitative test answers, and UCUM for quantitative values. The Unique Device Identification (UDI) system helps describe test methods and devices, though challenges remain for laboratory-developed tests (LDTs) and assays without UDIs. Combining standardised nomenclature and accurate data transmission is essential for achieving interoperability. The report demonstrates shortcomings in current interoperability by examining information exchange within and between two non-affiliated healthcare institutions for a specific set of laboratory test values for a single patient.
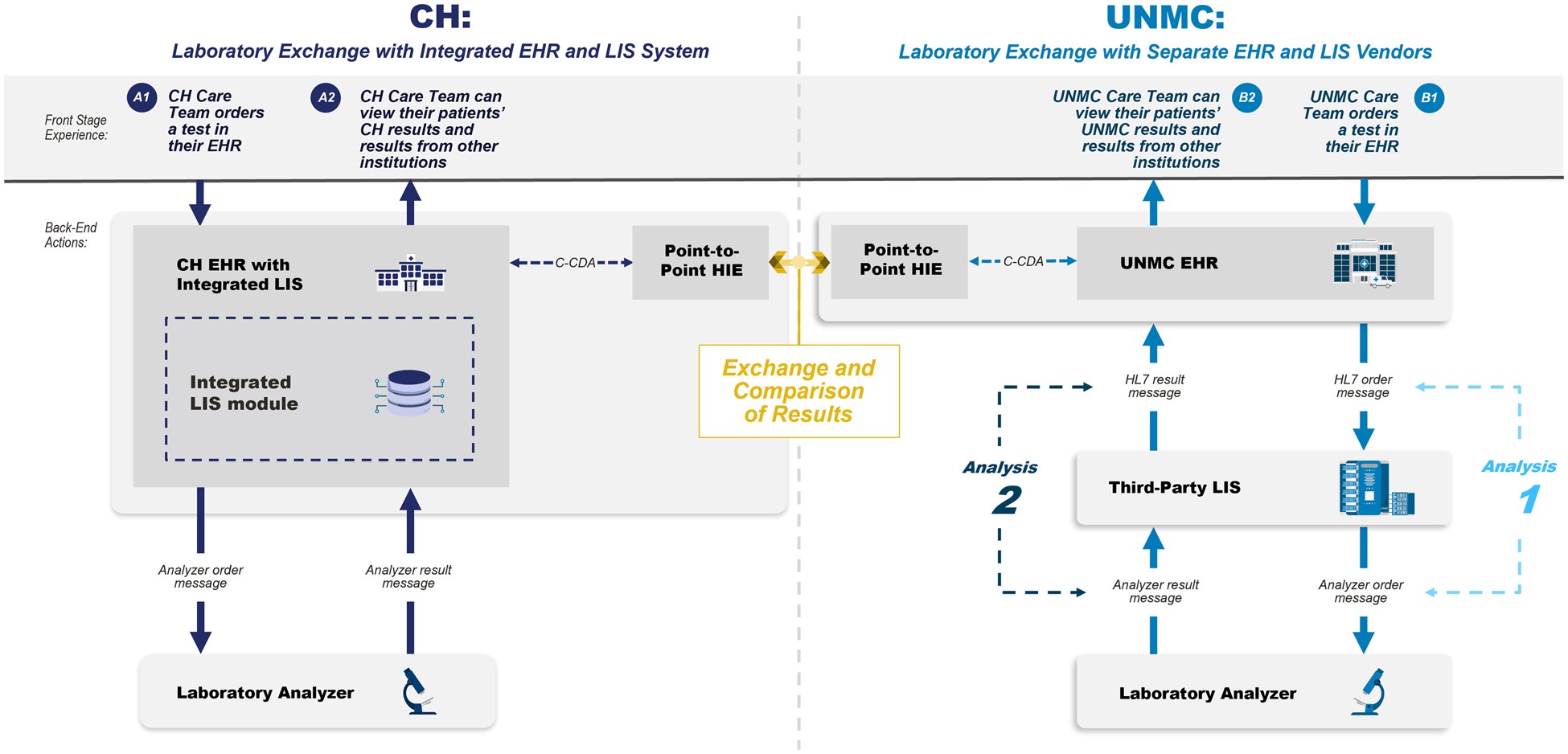
Image Credit: JAMIA OPEN
Interoperability Assessment between Major Healthcare Laboratories
The case report involves two major laboratories, the University of Nebraska Medical Center (UNMC) in Omaha, Nebraska, and Children’s Health (CH) in Dallas, TX. Both large healthcare facilities serve as reference testing centres for numerous other health facilities. They use Epic as their electronic health record (EHR) system. CH employs Epic Beaker as its laboratory information system (LIS), while UNMC uses CliniSys.
A simulated patient with identical identifiers was created in both EHRs. Nine clinically significant laboratory tests were selected, including quantitative and qualitative ones. Different instruments were used for testing at each location, except for the CBC.
Results were transferred between the two institutions using a common health information exchange (HIE) platform. Once received, they appeared in two locations within the receiving EHR. The completeness of displayed information was evaluated, including patient ID, report date, test performed, specimen source, result, units, reference range, abnormal flag, specimen exceptions, annotations, IVD instrument, method, and performing laboratory.
Messaging between the EHR, LIS, and analyzers was examined for consistency, utilising HL7 Version 2 messages for communication. However, one site used a local, non-standard dictionary for message transactions between the LIS and IVD instruments.
Bridging the Gap for Interoperability: The Need for Standardisation
The case report explores the challenges and implications of achieving interoperability in laboratory data exchange, focusing on the exchange between two major healthcare institutions. Despite recommendations from the Systemic Harmonisation and Interoperability Enhancement for Laboratory Data (SHIELD) and utilising Health Information Exchange (HIE) with standard messaging, achieving lab data interoperability remains elusive. Completeness of clinical laboratory data reporting, as governed by the Clinical Laboratory Improvement Amendments of 1988 (CLIA), is critical for patient safety. However, there are discrepancies in the transmission of essential data elements, such as specimen source, between different systems, potentially leading to misinterpretations and errors in medical treatment. Moreover, differences in analytical techniques, instruments, or platforms between institutions result in significant variability in reported test results, even for the same named test. Discordance in LOINC codes further complicates data interpretation and comparability.
Additionally, the use of multiple versions of HL7 within a single integration pattern exacerbates the issue, as it increases the complexity and effort required to ensure accurate data transmission. Addressing these challenges requires not only adherence to exchange standards like SHIELD recommendations but also standardisation of EHR and LIS functionality to capture, transmit, and display essential data elements consistently. Potential patient safety issues persist without these improvements, particularly when aggregating data across multiple laboratory sources.
Despite the importance of achieving interoperability in laboratory data exchange, there are few government mandates addressing the lack of standards in reporting laboratory data. Without standardised practices, assumptions about the comparability of results from different laboratories can lead to misunderstandings and errors in clinical decision-making. The study's limitations include its focus on relatively low complexity tests, all FDA-cleared assays, and the selection of institutions using the same EHR system. The challenges documented in this study are expected to worsen when exchanging data between heterogeneous systems and with more complicated tests and results.
Source: JAMIA Open
Image Credit: iStock