ICU Management & Practice, Volume 24 - Issue 3, 2024
Alarm fatigue is a pressing clinical problem in our post-pandemic ICUs and can adversely impact patient outcomes. Its root causes can be classified by patient, device and organisation related. We believe it can be mitigated and we propose interventions through attention to policy, education and the creation of a meaningful culture of safety.
"There are more things to alarm us than to harm us, and we suffer more often in apprehension than reality"
Seneca the Elder
Introduction
In 1983, there were an average of six different alarms in the ICU (Kerr and Hayes 1983). Fast forward 30 years, and this has exploded to over 40 potential monitors (Borowski et al. 2011). Continuous physiological monitoring of critically ill patients is now fundamental to healthcare provided in settings such as intensive care units, emergency departments, and operating rooms. Vital sign monitors are regarded as the sine qua non of advanced, safe, critical care. In short, they are critical to how we triage care and the numbers they display are afforded equal importance as the physical exam and laboratory findings. In addition to providing raw numbers such as heart rate, respiratory rate and blood pressure, continuous waveforms also provide surrogate measures of cardiac contractility and compliance, and biochemical health. In the event of significant variance from the “normal” physiology, these monitors will alert the bedside clinician, or others remotely, using auditory and visual alarms, and even text messages and emails. Electronic monitors have become our sentinels- in that they are on guard, minute by minute, for each of our patients- a level of vigilance that would be hard for any healthcare professional to maintain and to offer the potential to recognise early warning signs of acute deteriorations. Sometimes, the presence of such levels of monitoring has unfortunately and mistakenly been used to supplant patient reassessments through repeated physical exams and clinical acumen. Indeed, the word “monitor” itself comes from the Latin “to warn”. But do they achieve this goal? Are they, and should they be heeded?
This seemingly constant desire to monitor more and more is not without consequences. For some patients in intensive care settings, the number of alarm signals may exceed nine hundred per day (Graham and Cvach 2010). Each time an alarm is activated, the bedside clinician is interrupted from their current task and required to 1) identify which patient is affected, 2) identify which device is alarming; 3) determine if it is critical, urgent or non-urgent; 4) establish if it is actionable or non-actionable; and 5) pinpoint the type of action required. This interruption causes lasting distraction and loss of focus. As more hospitals become completely digitised, the possibilities of new alarms reflecting better integration of deteriorating physiological and laboratory parameters as part of early warning systems (EWS) are only increasing. Artificial intelligence (AI) may refine such alarms and increase the frequency of only actionable alerts. However, such sophisticated engagement with AI will take time to develop and will present its own adverse consequences.
Estimates are that more than 85-95% of alarms in the intensive care unit are non-actionable, “false alarms” (Ruppel et al. 2018). We might alternatively use the term “false alarm” in such situations. In everyday life, this expression usually comes with a sense of relief and reassurance that there is no fire or burglar, and in hospital settings, there is no actual code blue. Of note, the frequency range of alarms from a patient’s monitor in the ICU (2.5–3.15 kHz) is similar to a human scream or a baby’s cry (Derbyshire et al. 2019). This is deliberate in its design. It grabs our attention by triggering a very human reaction of cognitive distress; a rapidly increased state of arousal, and a quick response time (Ruskin and Heuske-Kraus 2015). This barrage of non-actionable alarms and emotional escalation and de-escalation inevitably causes healthcare practitioners to develop defence mechanisms. We can become desensitised, mistrusting, and, to a degree, apathetic towards the alerts. Together, this is called alarm fatigue, and it can have serious consequences on patient outcomes.
Despite many putative benefits associated with monitoring, more is not always more. While this article acknowledges the benefits of monitoring, our goal is to discuss alarm fatigue, its causes, and the challenges it poses in our post-pandemic ICUs, and to suggest ways in which its adverse effects can be mitigated moving forward.
Alarm Fatigue’s Impact on Patients and Healthcare Providers
Over the last decade, there has been increasing interest in alarm fatigue as a risk to patient safety and occupational health. As such, it is a high-priority issue for healthcare organisations (Sendelbach and Funk 2013). This prioritisation of alarm management has been helped by the Joint Commission’s (JCAHO) National Patient Safety Goals (NPSG) on alarm management. First issued in 2013 (Joint Commission, 2013), with its phase II released in 2016, the Joint Commission developed guidelines on alarm management after it issued a Sentinel Event Alert for 98 alarm-related incidents between January 2009 and June 2012. Of these events, 80 resulted in death, 13 in permanent loss of function, and five in unexpected additional care or extended stays. The Commission found that these sentinel events represent less than 10% of the actual alarm-related harms that occurred in hospitals.
Excessive alarms leading to alarm fatigue is associated with a prolonged length of hospital stay, increased morbidity and even increased mortality. The proposed mechanisms for this impact are the failure to respond to “true positive” alarms, due to desensitisation/deactivation of alarms, and decreased team performance, due to distraction and delays in patient care. For example, each interruption in a medication-related task is estimated to increase the probability of an error (after resumption) by 25% (Westbrook et al. 2010).
Following the COVID-19 pandemic, ICUs around the world have had to recruit large numbers of physicians, nurses, and inter-professional staff. Many of these new team members are starting their careers, in the ICU, rather than spending an initial period gaining experience in other hospital wards or ER first. This may lead to higher initial response rates to alarms. However, there is also a risk is that these staff fail to recognise truly critical alarms. In other words, they are less likely to separate signals from noise. For new ICU team members, there is a natural and normal experiential gap that means you “are not as attuned to what is deserving of attention” and you “do not yet know what you do not yet know”. Alarm fatigue is dangerous, especially when compounded by failure to escalate, professional insecurities, poor staff ratios, immature team culture, and unfamiliar team members and patient variables (Ede et al. 2019; Ede et al. 2021; O’Neil et al. 2021).
Aetiology of Alarm Fatigue
Many factors contribute to the clinical alarm burden and to the development of alarm fatigue in the intensive care unit (Figure 1).
Patient-related factors
Amongst monitored patients in an ICU, two-thirds of alarms are generated by one quarter of the patients (Schondelmeyer et al. 2018), and as many as 77% of false alarms are generated by as few as 2% of the patients (Harris et al. 2017).
Perhaps the most important factor in the ICU alarm burden is patient acuity. Units with higher acuity will incorporate more medical devices and expect a higher degree of vigilance. Therefore, patients with cardiac and respiratory failure, and especially those requiring mechanical ventilation, have more false alarms and generate more alarm fatigue. Patients who are older than 70 years and confused and agitated will often move constantly. This becomes a significant cause of waveform disturbances, and this increases the frequency of cardiac rhythm and pulse oximetry alarms (Schondelmeyer et al. 2018).
Responding to the right alarms requires skill and judgement and, despite having both, we are still prone to suffer from anchoring biases. For example, we may assume alarms are solely due to a patient’s confusion and agitation while failing to recognise the root cause of a patient’s confusion and agitation. In other words, alarm fatigue can delay timely patient care. Furthermore, our previous responses to alarms, whether they were assessed to be actionable or not (rightly or wrongly), and whether appropriate support and action were provided by physician team members when needed are likely to impact our future responses. In other words, once we develop alarm fatigue and failure to recognise and rescue, it could become a self-perpetuating habit.
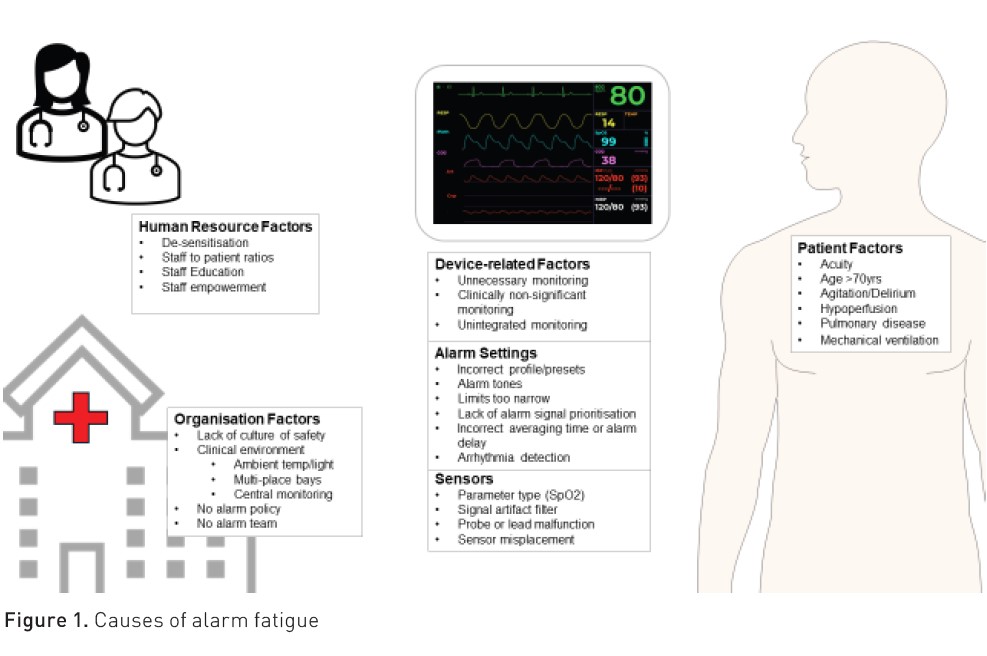
Device-related factors
Ideally, medical devices should have high diagnostic specificity and high positive predictive values (Cvach 2012). In other words, there should be few false positives or a few false “false” alarms. However, manufacturers of life-support systems, such as ventilators and monitoring systems, are under pressure to ensure that their products meet industry standards and they minimise legal liabilities with respect to any potential failure to alert critical incidents. As such, alarms are highly sensitive and tend towards over rather than under alert. Consequently, they will have low specificity and low positive predictive value.
The sound that comes from medical devices should "speak" to the clinician and inform them of the issue, its priority and its severity; unfortunately, frequently, they do not. Intensive care physiological monitors usually present with four or more different alert tones based on the importance of the issue. These alarm level sequences escalate in importance, for example: “message”, “advisory”, “warning”,” and critical”. However, the “language” is specific to the manufacturer and device. Furthermore, devices at the bedside are rarely integrated and hence frequently “speak out of turn”. Overlapping melodies reduce the listener’s ability to discriminate (Lacherez et al. 2007). This can mean, for example, the ventilator is not automatically prioritised before the less important “nagging” feeding pump.
International safety standards must be met by medical devices, and this includes their alarm systems. The International Electrotechnical Commission first produced a standard for alarm systems in medical equipment in 2003. Its current iteration, IEC 60601-1-8, which was amended in 2012, is a comprehensive set of technical specifications for safety and performance requirements. It addresses the volume, pitch, duration, repetition, and priority of alarms in medical equipment. However, “standard requirement” is not synonymous with standardisation, nor is compliance with the IEC standard mandatory. Therefore, clinical staff transferring from one hospital ward to another or from one monitoring system to another often need to learn to “translate a new language” to safely care for their patients (Edgworthy et al. 2014).
We also need to consider that device sensors, electrodes, and cables. Improper sensor placement, or expired electrode pads, will increase impedance of the electrical signal, which can affect waveform measurement and analysis. Even simple interventions such as daily electrode changes, or the location of blood pressure cuff and the oxygen saturation sensor can affect alarm incidence by nearly half (47%).
The fundamental electrical component inside an invasive pressure monitoring system is the Wheatstone bridge in its transducer. Movement of the transducer will lead to “noise” interference of the waveform. This is often easy to identify. However, a corroded cable connection will dampen the electrical signal, causing lower amplitude waveforms, which underestimates the pressure. These errors all generate technical alarms which require a reaction (movement of sensor, or replacement of an electrode or cable). These are highly prevalent (Wilken et al. 2017).
Environmental factors
Excessive ambient noise in hospitals has been increasing steadily by 0.26dB annually (Busch-Vishniac et al. 2007). In ICU it can adversely affect patient outcomes, whether through sleep disruption, increased sedatives, and/or increased delirium. The World Health Organization (WHO) and the U.S. Environmental Protection Agency (EPA) have advocated that sound levels in hospitals be limited to 45 dB during the day and 35 dB at night. However, neonatal ICUs have average sound levels of 48–61 dB for up to 95% of the day, paediatric units average 53–73 dB, and adult ICUs are 53–59 dB (Derbyshire et al. 2019). A significant proportion of this noise is from monitor alarms, typically located within 50cm of a patient's ears and producing default volumes greater than 50 dB. Moreover, as the important work by Derbyshire et al. (2019) has shown, the level of such noise can be experienced across the ICU setting (Figure 2).
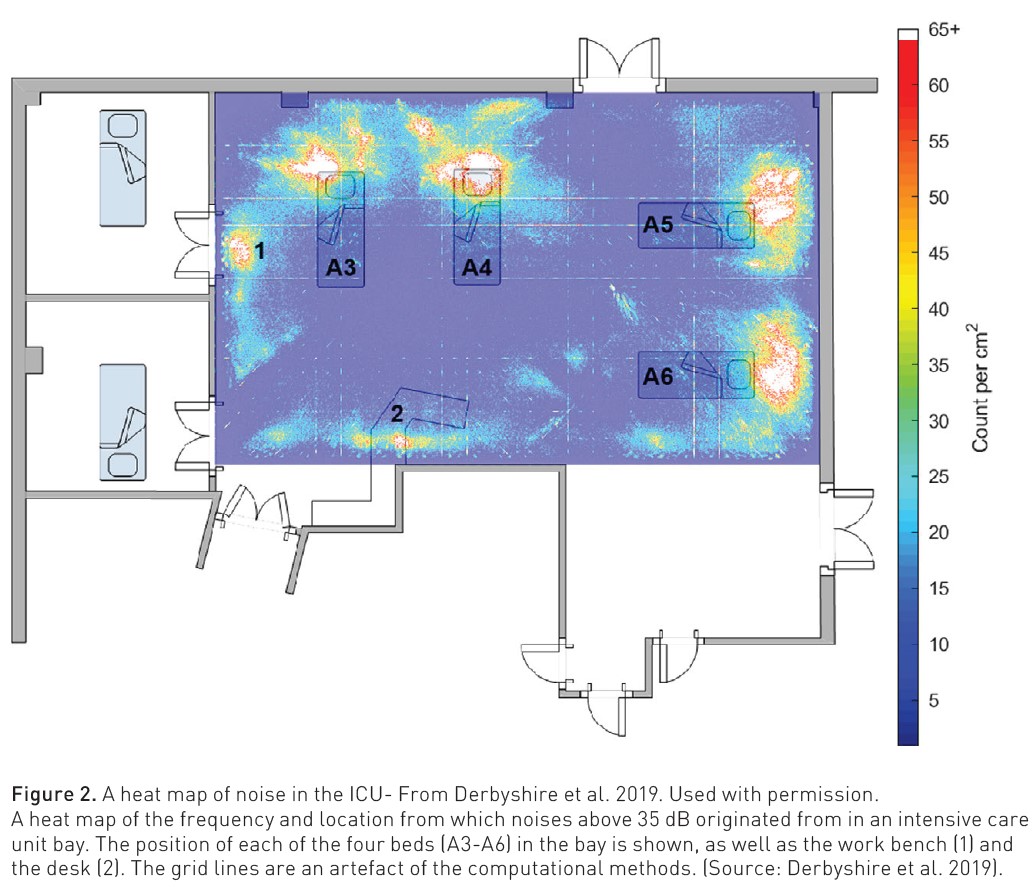
A heat map of the frequency and location from which noises above 35 dB originated from in an intensive care unit bay. The position of each of the four beds (A3-A6) in the bay is shown, as well as the work bench (1) and the desk (2). The grid lines are an artefact of the computational methods. (From Derbyshire et al, 2019).
The design of the ICU design has an independent impact on alarm fatigue. Clearly those cared for in multi-person rooms or bays will be exposed to greater ambient noise from other patient’s monitors, devices of alarms. Any delays in pinpointing what alarm is sounding and from which patient may have critical consequences in such environments. Wherever possible, central monitoring stations, remote from the bedside, mitigate some of this noise pollution for patients, families and healthcare teams.
Organisation-related factors
Organisational policy also significantly influences the incidence and exposure to alarms in healthcare environments. Broadly speaking, the risk of alarm fatigue can be mitigated by a hospital’s patient staffing models, training programmes and through the development of an institutional alarm policy.
The most important organisation-related factor for alarm fatigue is the empowerment of clinical staff to adjust alarm thresholds to their patient’s clinical situation. Monitors usually have default setting profiles that are programmed by manufacturers. Many monitors also allow for defined profiles better suited to a patient's age, condition, or physiology. The probability of false alarms increases if the wrong profile is used, for example, adult defaults used for a paediatric patient or not adjusted to a patient's baseline values.
Organisations should develop formal clinical monitoring policies that clearly define the scope and circumstances where staff are safe and empowered to make changes and where this is dangerous and hence forbidden. Incorrect alarm limits affect their sensitivity, which can delay the recognition of a critical deterioration. This is particularly true when alarms are silenced because we assume the patient will remain stable. Any alarm policy should be tailored to the staff and promote their education and their understanding of medical devices and alarm management. As primary operators of monitoring systems, ICU nurses need adequate training and ongoing user support. However, research suggests that 60% of ICU nurses may have received insufficient monitoring training to properly manage alarms (Sowan et al. 2016). Initial training and ongoing support have been shown to improve alarm setting complianceand decrease the alarm burden (Brantley et al. 2016). Furthermore, any alarm policy should be linked to escalation of care policies to identify who should be called for help.
Where Do We Go From Here?
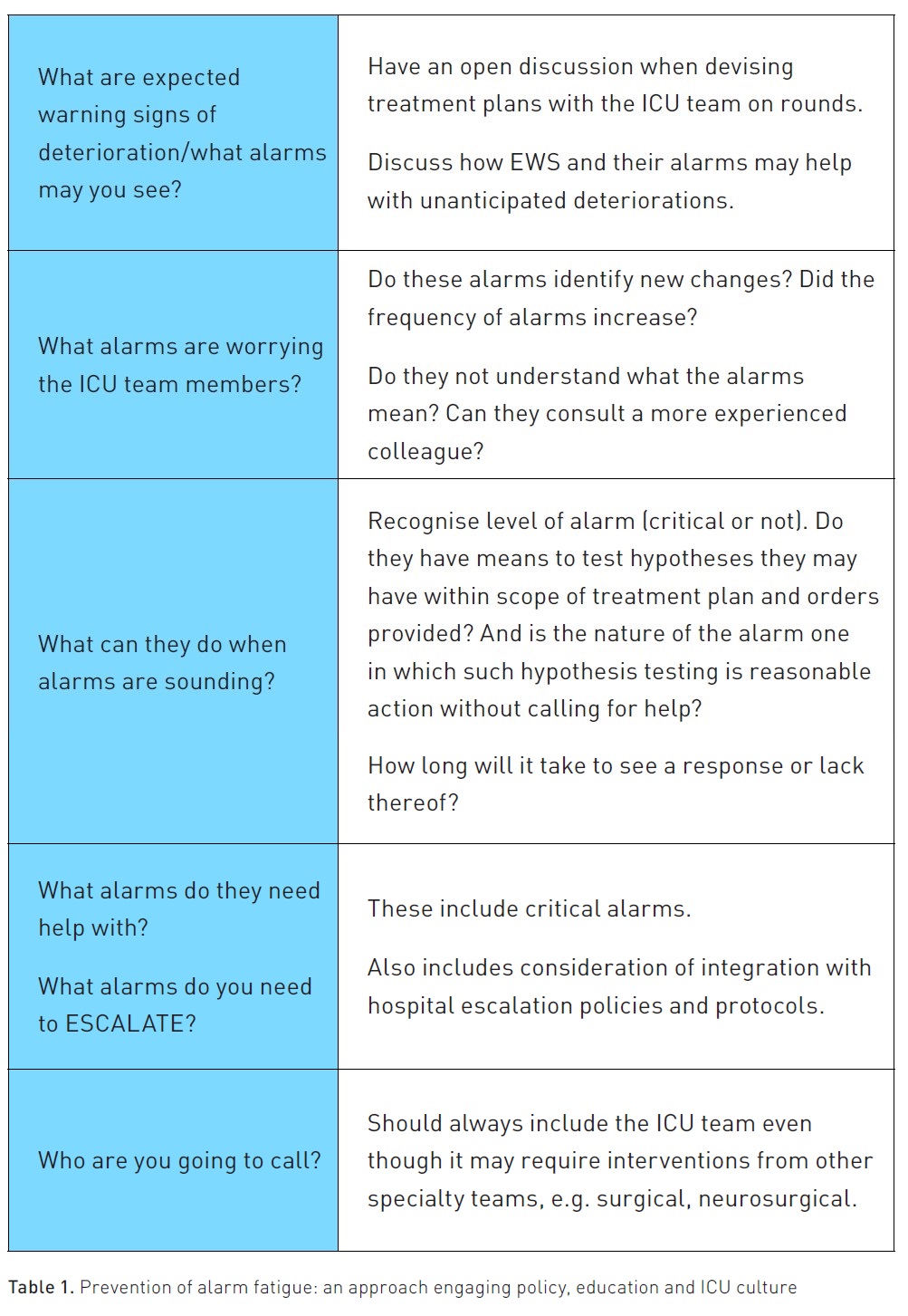
There are opportunities for change that can improve patient outcomes, and arguably, such change is needed now, following the recent pandemic, more than ever. These include developing and finessing hospital alarm policies and providing deliberate team-based education on both alarm systems and the causes and risks of alarm fatigue. Yet such measures may not be enough. Hospitals and ICU teams also need to create a culture of safety wherein if any team member is unsure of the significance of an alarm, they feel supported when they raise or escalate their concern and that dealing with alarms is a team responsibility, not just that of the bedside nurse or registered respiratory therapist. This culture shift can be encouraged by (Table 1):
- All ICU team members being aware of what signs of deterioration may occur with any given patient and how EWS bolsters recognition of unanticipated deteriorations.
- Encouraging team members to discuss any signs/alarms that worry them, even if they are uncertain or don't understand their significance.
- Ensure all team members understand how to respond to alarms within their scope of practice. In other words, the freedom they have in accordance with treatment plans and/or existing orders and how long they have to establish if their hypothesis seems correct.
- Ensure they can identify when they need help, i.e. when they need to escalate responses to alarms.
- Ensure they understand who they need to call and when such calls must occur (e.g. when alarms are critical).
The equipment industry needs to meet IEC standards. Yet, such standards should also continuously evolve to meet patient and clinician needs. Industries have been concerned about alarm fatigue for some time and are already exploring the role of AI moving forward. It is time for us clinicians to be similarly engaged.
Conclusion
Alarm fatigue is a serious issue in the care of critically ill patients and is widely accepted as an independent factor that significantly and negatively impacts patient outcomes. It is of greater concern to us now, in this post-pandemic era, as we introduce, onboard, and train so many new team members. Alarm fatigue is multifactorial and is affected by patient, device, environment, and organisational factors. Fortunately, many of these factors can be addressed within an ICU through policy change, education, and the promotion of a supportive culture of safety. There is hope for the future in mitigating alarm fatigue, but only if we tackle it as an inter-professional ICU team alongside our industry partners.
Conflict of Interest
None.
References:
Borowski M, Gorges M, Fried R et al. (2011) Medical device alarms. Biomed Tech (Berl). 56(2):73-83.
Brantley A, Collins-Brown S, Kirkland J et al. (2016) Clinical trial of an educational program to decrease monitor alarms in a medical intensive care unit. AACN Crit Care. 27(3):283-289.
Busch-Vishniac I, West JE, Kwon P, Dunn J (2007) The challenges of noise control in hospitals. 14th International Conference on Sound and Vibration. Cairns, Australia. 1-13.
Cvach M (2012) Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 46(4):268-277.
Derbyshire JL, Muller-Trapet M, Cheer J et al. (2019) Mapping sources of noise in an intensive care unit. Anaesthesia. 74:1018-1025.
Ede J, Petrinic T, Westgate V et al. (2021) Human Factors in escalating acute ward care: a qualitative evidence synthesis. BMJ Open Qual. 26;10(1).
Ede J, Jeffs E, Vollam S, Watkinson P (2019) A qualitative exploration of escalation of care in the acute ward setting. Nurs Crit Care. 25(3):171-178.
Edworthy J, Page R, Hibbard A et al. (2014) Learning three sets of alarms for the same medical functions: a perspective on the difficulty of learning alarms specified in an international standard. Appl Ergon. 45:1291-1296.
Graham K, Cvach M (2010) Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 19(1):28-35.
Harris PR, Zègre-Hemsey JK, Schindler D et al. (2017) Patient characteristics associated with false arrhythmia alarms in intensive care. Ther Clin Risk Manag. 13:499-513.
Johnson KR, Hagadorn JI, Sink DW (2017) Alarm Safety and Alarm Fatigue. Clin Perinatol. 44:713-728.
Joint Commission (2013) Sentinel event alert issue 50: medical device alarm safety in hospitals. April 8.
Kerr JH, Hayes B (1983) An ‘‘alarming’’ situation in the intensive therapy unit. Intensive Care Med. 9(3):103-104.
Lacherez P, Seah EL, Sanderson P (2007) Overlapping melodic alarms are almost indiscriminable. Hum Factors. 49:637-645.
O’Neil SM, Clyne B, Bell M et al. (2021) BMC Emerg Med. 21(1):15.
Ruppel H, Funk M, Whittemore R (2018) Measurement of physiological monitor alarm accuracy and clinical relevance in intensive care units. Am J Crit Care. 27(1):11-21.
Ruskin KJ, Hueske-Kraus D (2015) Alarm fatigue: impacts on patient safety. Curr Opin Anaesthesiol. 28(6):685-690.
Schondelmeyer AC, Brady PW, Goel VV et al. (2018) Physiologic monitor alarm rates at 5 children's hospitals. J Hosp Med. 13(6):396-398.
Sendelbach K, Funk M (2013) Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 24(4):378-386.
Steurer R (2016) Chairperson Association for the Advancement of Medical Instrumentation (AAMI) Standards Committee, Taxonomy Workgroup. September 12, personal communication.
Sowan AK, Gomez TM, Tarriela AF et al. (2016) Changes in default alarm settings and standard in-service are insufficient to improve alarm fatigue in an Intensive Care Unit: a pilot project. JMIR Hum Factors. 3(1):e1.
Westbrook JI, Coiera E, Dunsmuir WT et al. (2010) The impact of interruptions on clinical task completion. Qual Saf Health Care. 19:284-289.
Wilken M, Huske-Kraus D, Klausen A et al. (2017) Alarm fatigue – causes and effects. In: Rohrig R et al. (Eds). German Medical Data and Sciences: Visions and Bridges. IOS Press.