ICU Management & Practice, Volume 24 - Issue 1, 2024
Evidence regarding the impact of the ICU pharmacist demonstrates improved outcomes in ICU patients, with a significant impact on the cost of care. This evidence comes from multiple studies performed in different countries. What happens in Europe at the moment? This article describes clinical pharmacy services in a number of Intensive Care Units across Europe.
Historical Perspectives from the U.S. and the U.K.
Patients admitted to Intensive Care Units (ICU) receive multiple medications, mostly by the intravenous route, requiring careful calculation, initiation and follow-up to ensure efficacy and safety. They have rapidly changing medical conditions and organ function, laboratory values and medication. They are more vulnerable to medication errors (ME) and associated adverse drug events (ADE) due to the complexity and intensity of pharmacotherapy (Kane-Gill et al. 2010; Bosma et al. 2021). ME in critically ill patients are most often caused by patient-related factors (e.g. poor physiological reserve, which potentially increases the risks of harm from medication-related errors), medication factors (polypharmacy, including high-risk medications) and dosing- and administration related problems (mainly prescription of continuous infusions, where the doses are calculated according to patient's weight, renal and hepatic functions). Besides these factors, errors are also caused by drug shortages, frequently occurring in the ICU (McKenzie et al. 2024).
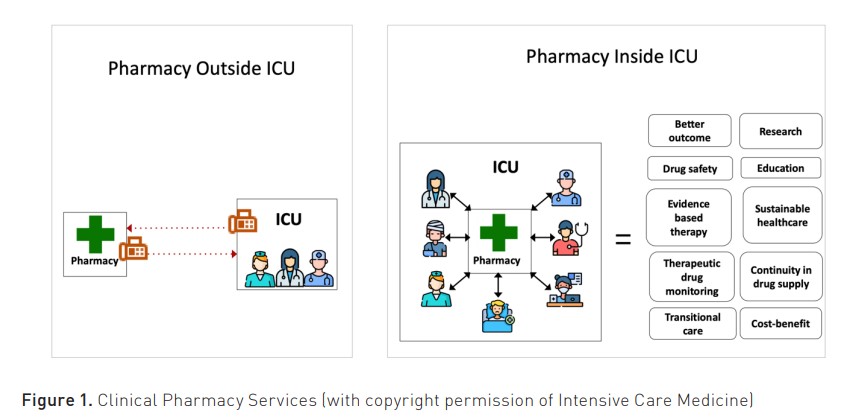
Optimising medication is a central and key role expected of hospital pharmacists in all clinical areas, not only in intensive care medicine (Bourne et al. 2022). Ideally, all critically ill patients should have input from a suitably trained critical care pharmacist. Critical care pharmacists add value in a range of different ways, e.g. through direct patient care (pharmacist-driven protocols, medication review and monitoring), involvement in the critical care multidisciplinary ward rounds, and, of course, in the development of clinical guidelines, patient safety initiatives, financial management and quality improvement initiatives (Newsome et al., 2021). Figure 1 nicely illustrates the key roles of bedside ICU pharmacists.
Pharmacists became involved in critical care in the U.S. starting in the 1960s following the publication of several studies linking their role to improved outcomes and reduced costs (Chant et al. 2015). Leape et al. (1999) published a landmark paper and clearly demonstrated that the presence of pharmacists in a medical ICU reduced medication errors and associated costs. A large survey conducted in U.S. hospitals in 2004 compared costs and clinical outcomes in ICUs with at least a part-time dedicated pharmacist to those without. It showed improved costs and clinical outcomes, i.e. a shorter ICU length of stay and lower hospital mortality, particularly in patients with infections (MacLaren et al. 2008) and thrombo-embolic diseases (MacLaren and Bond 2009).
In the U.K., in the 1960s and 70s, an initiative called "ward pharmacy" was developed in response to concerns regarding errors in the administration of medication to patients in hospitals. Patient-specific drug charts and the deployment of pharmacists to wards were among the early initiatives aimed at streamlining the supply of medication and improving patient safety. Pharmacists also started to develop their consultative role and gradually became a source of information for medical and nursing teams. As the 'ward-pharmacist role' expanded over the next 25 years, new services like therapeutic drug monitoring and drug information services developed, and some of these new roles were called "clinical pharmacy services" (Cotter 1995).
This often sporadic involvement in the U.K. health system in the 90s evolved towards a more overt, in-depth role formally described by the NHS Modernisation Agency in 2002 (NHS Modernisation Agency 2002) and ‘Adult Critical Care: Specialist Pharmacy Practice’ was published by the British Department of Health in 2005 (Department of Health England 2005). The publication of ‘Core standards for intensive care units’ in 2013 and subsequent incorporation into ‘Guidelines for the Provision of Intensive Care Services’ in 2016 further established this. The latest update, GPICS v 2.1 (2022), is the definite reference source for planning and delivery of U.K. Critical Care Services and includes specific recommendations related to staffing levels in critical care for pharmacy staff.
In 2020, data was collected regarding ICU pharmacy staffing at U.K. critical care units. Data was obtained for 96% of U.K. critical care units, and it found that 98.2% of U.K. critical care units had specific clinical pharmacist time dedicated to the unit (Borthwick et al. 2023).
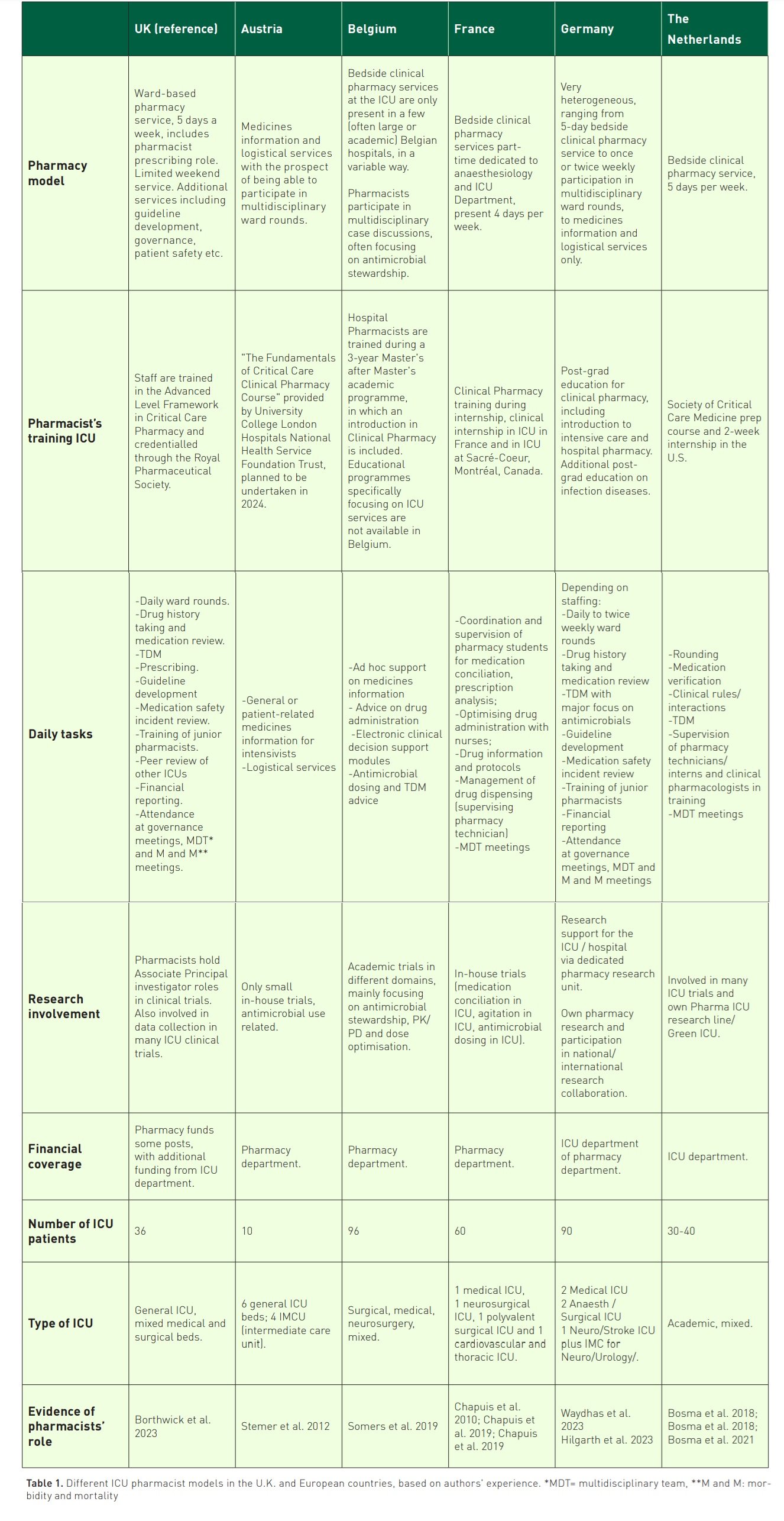
But what happens in ICUs throughout Europe? Can pharmacists in European countries achieve what is shown in the U.S. and the U.K., i.e. reduction of medication errors and costs? This paper describes the different pharmacy models used by ICU pharmacists from the U.K. (reference country), Austria, Belgium, France, Germany and the Netherlands. We also show the existing national evidence about ICU pharmacists and finish with future perspectives.
ICU Pharmacist Involvement Throughout Europe
In Europe, different pharmacy models are used in ICUs, ranging from pharmacists providing services from the pharmacy (often "the basement" or "back-office") to full availability at the ICU ward itself (bedside or "front-office"). Information from ICU pharmacists from different countries in Europe and the U.K. (reference country) is provided in Table 1. It shows which pharmacy models (pharmacy, ward or mixed) are used, how pharmacists are trained, what their tasks are, how they are involved in research and how their role is covered financially. Furthermore, information about the format, type and size of the ICU is provided, together with evidence about the impact of the ICU pharmacist.
Austria
The implementation of Intensive Care Clinical Pharmacy Services in Austria exhibits considerable heterogeneity not only across different counties but also among individual healthcare trusts. The funding mechanisms for these positions also manifest notable diversity among care providers, predominantly originating from hospital pharmacy departments. The absence of legal regulations governing clinical pharmacy services in Austria grants considerable autonomy to the operational trusts of medical facilities, allowing them to determine the extent and quantity of clinical pharmacists within their employ.
Currently, there is no formal national training programme for ICU clinical pharmacists available in Austria. However, clinical pharmacy training is an integral component of the broader hospital pharmacy specialisation, comprising 80 units out of a total of 240 units in the curriculum (Österreichische Apothekerkammer: Weiterbildungsordnung - Krankenhausfachapotheker (KhFA-WbO 2015 idgF.). Additionally, a postgraduate Master of Science (MSc) degree programme in clinical pharmacy has been implemented at the University of Vienna since autumn 2023 (Klinische Pharmazie 2024). According to a national survey conducted in 2020, 18.8% of pharmacists employed in Austrian hospitals had successfully completed a postgraduate MSc degree in clinical pharmacy practice, either in the U.K. or elsewhere (Deibl et al. 2020). Anecdotal evidence suggests that some critical care clinical pharmacists have pursued specialised courses, such as the “Fundamentals of Critical Care Clinical Pharmacy Course” provided by University College London (The Fundamentals of Critical Care Clinical Pharmacy 2024).
Notably, not every hospital in Austria that provides critical care maintains a pharmacy department or offers clinical pharmacist positions, as evidenced by the fact that only 15.8% of Austrian hospitals house a Pharmacy Department (Österreichische Apothekerkammer: Apotheke in Zahlen 2020) with even fewer providing clinical pharmacy services. Clinical pharmacists engaged in ICU service delivery in Austria report a spectrum of critical care models, ranging from on-call provision of medication information to the consistent delivery of services, including weekly participation in ward rounds or even more frequent engagement in bedside patient care activities.
The Netherlands
The presence of a pharmacist during the daily multidisciplinary ICU meetings is obligatory based on the Dutch ICU care guidelines (ZorgInstituut 2016). All Dutch pharmacy residents follow a one-day training about ICU pharmacology to be able to join the daily multidisciplinary ICU meetings. In 2012, Hunfeld and colleagues showed in a business case that the addition of a dedicated ICU pharmacist to the medical teams of the ICU resulted in a positive financial outcome. The data used in the business case were published in combination with data from an ICU in a large teaching hospital (Bosma et al. 2018). At the Erasmus Medical Center (MC), the data from the business case resulted in 0.5 FTE ICU pharmacists for (on average) 35 patients. Since there is no specific clinical training for ICU pharmacists in the Netherlands, knowledge and skills are obtained in a " learning by doing" format by sharing information with other ICU pharmacists and through literature from the USA and the UK. It is estimated that 10 out of 80 ICUs in the Netherlands have implemented Intensive Care Clinical Pharmacy Services with a dedicated ICU pharmacist. Funding comes from either the pharmacy or the ICU. With regard to research, many pharmacists are involved in ICU research, mostly focussing on PK/PD trials or medication safety (Wallenburg et al. 2021; Bakker et al. 2024).
Belgium
Clinical pharmacy is a relatively young discipline in Belgium, with the initiation of bedside clinical pharmacy services at different hospital wards, including ICUs, in only a few academic hospitals in 2003. Driven by the favourable results of these first projects, further development of clinical pharmacy services was funded from 2007 onwards in more than 50 acute care hospitals by the Belgian government. Simultaneously, the master’s degree in hospital pharmacy was expanded to a 3-year inter-university Master's after Master's programme, in which an educational programme addressing clinical pharmacy fundamentals is embedded, followed by an obligatory internship of 22 weeks at different hospital wards (Somers et al. 2019).
Due to limited national and hospital funding for hospital pharmacy services, clinical pharmacy is typically organised in a three-layered structure in Belgium. First, as all Belgian acute care hospitals are equipped with electronic health records, accurate prescribing is facilitated for hospitalised patients by embedding clinical decision support modules in the electronic prescribing system. These clinical decision support modules include alert systems for drug-drug interactions, maximum dosing, therapeutic duplication, drug allergies, serious adverse drug reactions and the use of teratogenic medication in pregnancy. Clinical pharmacists often contribute to the development and further fine-tuning of these systems, aiming at better performance (Van de Sijpe et al. 2022).
Second, some wards have a bedside clinical pharmacy service. Pharmacists are part of the clinical team and participate in ward rounds and multidisciplinary case discussions, contribute to the effective and safe use of drugs and counsel caregivers and patients. This is typically carried out by highly specialised pharmacists on wards where the risk of drug-related problems is high, i.e. geriatric, oncology, haematology, surgical, transplantation and ICU clinical areas (Somers et al. 2019).
Finally, due to limited funding to expand these bedside clinical pharmacy services, a new back-office service (often referred to as 'Check of Medication Appropriateness') was implemented in most Belgian hospitals. In this hospital-wide service, electronic medical records are screened continuously and in real-time for drug-related problems based on automated clinical rules embedded in the hospital information system. When a drug-related problem is identified by a clinical rule, a clinical pharmacist will review the patient's file and advise the treating physician or the nurse. This service leads to a high acceptance rate (Quintens et al. 2022) and satisfaction, and a significant reduction in the number of inappropriate prescriptions associated with patient harm and is now mandated by Belgian legislation (Quintens et al. 2022; Quintens et al. 2021).
Belgian pharmacists are often involved in academic research at the ICU, focusing on (antimicrobial) pharmacokinetics, optimisation of drug dosing and therapeutic drug monitoring (Dhont et al. 2023; Van Daele et al. 2022).
In general, the involvement of bedside clinical pharmacists in the daily management of patients admitted to the ICU is limited in Belgium. These services are only available in a few Belgian hospitals, and pharmacists are allocated to ward areas for a limited amount of time. A national overview of the Belgian ICU pharmacy professional workforce is not available. Ideally, international standards should mention pharmacy professionals as obligatory members of the ICU management teams. This would encourage hospital directors to reallocate pharmacy resources to the patients who need them the most. In parallel, Belgian clinical pharmacists should be trained in the fundamental knowledge and skills necessary to contribute effectively to the critical care team.
Germany
Clinical pharmacy services for intensive care units have developed steadily over the last ten years. However, considerable variation remains between the services offered across regions and hospitals. A recent German survey of intensive care departments showed that only 35% of the participants had a pharmacy service. Not surprisingly, intensivists with a clinical pharmacy service rated services such as ward rounds, medication reviews, management of ME, TDM, cost evaluation and management of adverse events alongside medical information and the availability of pharmacy consultation by telephone (24h) as essential. Clinicians without experience in a pharmacy service only rated medical information and the availability (24h) as essential. Core clinical tasks by pharmacists were seen as nice to have (Hilgarth et al. 2023). Therefore, it is not surprising that staffing levels range considerably, from limited or no funding and from providing a weekly ward round only to centres providing comprehensive clinical services.
The recent “Recommendations on the structure, personnel, and organisation of intensive care units”, published in 2022 by the German Association of ICU (DIVI), strongly advises providing a clinical pharmacy service during weekdays and out-of-hours. Importance is given to providing a twice-weekly ward round with a dedicated pharmacist (Waydhas et al. 2022). To comply with the recommendation, the DIVI suggests a pharmacist-to-bed ratio of at least 1:30 (0.033 FTE for one intensive care bed). However, robust data on staffing levels is rare. At the University Hospital Dresden, all adult intensive care units now have a clinical pharmacy service in place with dedicated trained pharmacists with a ratio 1:40 (0.025 FTE for one intensive care bed).
Despite the recent demonstration of the economic benefit of clinical pharmacy services in a German intensive care unit (Liebing et al. 2024), funding for dedicated pharmacy services remains one of the main barriers at the moment. However, the recommendation for a pharmacy service will undoubtedly have an impact in the coming years.
The collaborative work between the DIVI and German Hospital Pharmacist Association (ADKA) leads to results. Pharmacists are regular presenters at the yearly DIVI congress, sharing their experience and research. There has been active work in sections of the DIVI. Work on standardising infusion practice has been published (Kraemer et al. 2023), and work on catheter compatibilities and syringe labelling is ongoing.
Where some pharmacy departments, like Dresden, now have dedicated pharmacists with over ten years of experience in critical care, staff training remains a challenging issue for most centres starting new services. There has been recent work in providing additional post-graduate education on pharmaceutical care, including aspects of basics in critical care. The ADKA critical care pharmacy group offers advanced training twice a year. However, this can only be considered a starting point and comprehensive post-graduate training is required. There has been a rising interest and uptake in some courses offered abroad; however, a national/European career path (development) and peer support are required to strengthen the overall provision across centres. Both topics are currently discussed in the ADKA Pharmacy critical care groups.
France
In France, the practice of clinical pharmacy has been developing since the 1980s, with the creation of the French Society of Clinical Pharmacy in 1984, which has developed strong connections with the French Society of Anesthesia and Intensive Care Medicine. Clinical pharmacy was mandated as a core activity for hospital pharmacists in 2016. Clinical pharmacy is practiced in a highly heterogeneous way across the country. In intensive care, very few pharmacists have time allocated to clinical pharmacy as part of their job plan. There is no data on the number of pharmacists working in intensive care nor on the ratio of pharmacists per number of beds. Some hospitals have funded trials of ICU pharmacists working at the bedside, but these have not been continued owing to a lack of resources (Moch et al. 2014; Maison et al. 2020). Some pharmacists became involved in intensive care through their expertise in medical devices, particularly drug infusions (Négrier et al. 2021), while others are more directly involved in patient care, e.g. on targeted projects such as therapeutic drug monitoring of antibiotics (Correia et al. 2023). A few French teams have published their experience in the ICU based on a range of clinical pharmacy activities (Leguelinel-Blache et al. 2018; Lemtiri et al. 2020; Chapuis et al. 2019), demonstrating clinical and economic benefits for the management of intensive care patients.
Training in clinical pharmacy is developing, thanks to the Association Nationale des Enseignants de Pharmacie Clinique (National Association of Teachers of Clinical Pharmacy), but at present, no French programme specific to the intensive care environment is available. Only a few training modules are included in pharmacy curricula (e.g., the Grenoble-Geneva-Lausanne inter-university diploma in advanced clinical pharmacy practice includes a "critical care" day). Most of the few pharmacists working in intensive care (notably in the hospitals of Grenoble, Caen, Valenciennes, Saint-Etienne, Lille, Nîmes, Annecy and Montélimar) have been trained in general clinical pharmacy, and then specifically on-the-job while working in the ICU. Pharmacists can have access to medical intensive care training programmes, but it is not necessarily suitable. There is a need to develop specific training in the French language to further encourage pharmacists to get involved more and more in intensive care units.
Future Perspectives
A recent publication in Intensive Care Medicine (McKenzie et al. 2024) described the ten reasons why pharmacists and pharmacy technicians should be embedded in every ICU team, supported by evidence from multiple studies in different countries (Figure 1). The ultimate goal is to equip every ICU in Europe with an ICU pharmacist. This requires support from international societies, especially the International Society of Intensive Care and Emergency Medicine (ISICEM) and the European Society of Intensive Care Medicine (ESICM). Within ESICM, a group of ICU pharmacists are developing a white paper (position paper) which will update current recommendations for critical care pharmacy practice and make recommendations for the future. Furthermore, ICU pharmacist training and examination (like the European Diploma in Intensive Care Medicine (EDIC)) needs to be developed and implemented over the next few years. Most importantly, the incorporation of the ICU pharmacist in national ICU quality guidelines, together with financial support, is key. These are the necessary conditions to support the ICU pharmacist's transition from basement to bedside in Europe.
Acknowledgements
We thank Peter Glaves for his graphical design input.
Conflict of Interest
Nicole Hunfeld is leading the ESCH-R research project about circular hospitals (granted by the Dutch Research Council (NWO)). All other authors have no conflict of interest to declare. Isabel Spriet is supported by the Clinical Research Fund, University Hospitals Leuven, Belgium.
References:
Bakker T, Klopotowska J, Dongelmans D et al. (2024) The effect of computerised decision support alerts tailored to intensive care on the administration of high-risk drug combinations, and their monitoring: a cluster randomised stepped-wedge trial. Lancet. Epub ahead of print.
Borthwick M (2019) The role of the pharmacist in the intensive care unit. J Intensive Care Soc. 20(2):161-164.
Borthwick M, Barton G, Ioannides C et al. (2023) Critical care pharmacy workforce: a 2020 re-evaluation of the UK deployment and characteristics. Hum Resour Health. 21:28.
Bosma L, Hunfeld N, Quax R et al. (2018) The effect of a medication reconciliation program in two intensive care units in the Netherlands: a prospective intervention study with a before and after design. Ann Intensive Care. 8(1):19.
Bosma B, van den Bemt P, Melief P et al. (2018) Pharmacist interventions during patient rounds in two intensive care units: Clinical and financial impact. Neth J Med. 76(3):115-124.
Bosma B, van Rein N, Hunfeld N et al. (2019) Development of a multivariable prediction model for identification of patients at risk for medication transfer errors at ICU discharge. PLoS One. 14(4):e0215459.
Bosma B, Hunfeld N, Roobol-Meuwese E et al. (2021) Voluntarily reported prescribing, monitoring and medication transfer errors in intensive care units in The Netherlands. Int J Clin Pharm. 43(1):66-76.
Bourne R, Jennings J, Panagioti M et al. (2022) Medication-related interventions to improve medication safety and patient outcomes on transition from adult intensive care settings: a systematic review and meta-analysis. BMJ Qual Saf. 31(8):609-622.
Chant C, Dewhurst N, Friedrich J (2015) Do we need a pharmacist in the ICU? Intensive Care Med. 41:1314–1320.
Chapuis C, Roustit M, Bal G et al. (2010) Automated drug dispensing system reduces medication errors in an intensive care setting. Critical Care Med. 38(12):2275-81.
Chapuis C, Chanoine S, Colombet L et al. (2019) Interprofessional safety reporting and review of adverse events and medication errors in critical care. Ther Clin Risk Manag. 2;15:549-556.
Chapuis C, Albaladejo P, Billon L et al. (2019) Integrating a pharmacist into an anaesthesiology and critical care department: Is this worthwhile? Int J Clin Pharm. 41(6):1491-1498.
Correia P, Launay M, Balluet R et al. (2023) Towards optimization of ceftazidime dosing in obese ICU patients: the end of the 'one-size-fits-all' approach? J Antimicrob Chemother. 78(12):2968-2975.
Cotter SM (1995) The role of the hospital pharmacist in the United Kingdom National Health Service (PhD thesis). London: University of London (London School of Hygiene & Tropical Medicine).
Deibl S, Mueller D, Kirchdorfer K et al. (2020) Self-reported clinical pharmacy service provision in Austria: an analysis of both the community and hospital pharmacy sector-a national study. International Journal of Clinical Pharmacy. 42(4):1050–1060.
Department of Health (London, England) (2005) Adult critical care specialist pharmacy practice.
Dhont E, Van Der Heggen T, Snauwaert E et al. (2023) Predictors of augmented renal clearance based on iohexol plasma clearance in critically ill children. Pediatr Nephrol. Epub ahead of print.
Hilgarth H, Waydhas C, Dörje F et al. (2023) Arzneimitteltherapiesicherheit gefördert durch die interprofessionelle Zusammenarbeit von Arzt und Apotheker auf Intensivstationen in Deutschland. Med Klin Intensivmed Notfmed. 118:141-148.
Kane-Gill S, Jacobi J, Rothschild J (2010) Adverse drug events in intensive care units: risk factors, impact, and the role of team care. Crit Care Med. 38(6 Suppl):S83-9.
Klinische Pharmazie (2024) Available at https://www.postgraduatecenter.at/weiterbildungsprogramme/gesundheit-naturwissenschaften/klinische-pharmazie/
Kraemer I, Kreysing L, Hilgarth H et al. (2023) Empfehlungen zu Standardkonzentrationen für die kontinuierliche Infusion auf Intensivstationen (Standardkonzentrationsliste Dauerinfusionen). Krankenhauspharmazie. 44: 393–399.
Leape L, Cullen D, Clapp M et al. (1999) Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 282(3):267–270.
Leguelinel-Blache G, Nguyen T, Louart B et al. (2018) Impact of Quality Bundle Enforcement by a Critical Care Pharmacist on Patient Outcome and Costs. Crit Care Med. 46(2):199-207.
Lemtiri J, Matusik E, Cousein E et al. (2020) The role of the critical care pharmacist during the COVID-19 pandemic. Ann Pharm Fr. 78(6):464-468.
Liebing N, Ziehr B, Rober S et al. (2024) Ward-based clinical pharmacists in intensive care medicine: an economic evaluation. Med Klin Intensivmed Notfmed.
MacLaren R, Bond C, Martin S et al. (2008) Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 36(12):3184–3189.
MacLaren R, Bond C (2009) Effects of pharmacist participation in intensive care units on clinical and economic outcomes of critically ill patients with thromboembolic or infarction-related events. Pharmacotherapy. 29(7):761–768.
Maison O, Tardy C, Offrey J et al. (2020) Compliance with sedation analgesia protocols: Do clinical pharmacists have an impact? J Clin Pharm Ther. 45(1):59-64.
McKenzie C, Spriet I, Hunfeld N (2024) Ten reasons for the presence of pharmacy professionals in the intensive care unit. Intensive Care Med. Epub ahead of print.
Moch C, Pivot C, Floccard B et al. (2014) Intégration d'un pharmacien hospitalier en service de réanimation [Integration of a hospital pharmacist in the ICU]. Ann Pharm Fr. 72(2):90-4.
Négrier L, Martin Mena A, Lebuffe G et al. (2021) Strategies to prevent drug incompatibility during simultaneous multi-drug infusion in intensive care units: a literature review. Eur J Clin Pharmacol. 77(9):1309-1321.
NHS Modernisation Agency 2002 Critical care programme: AHP and HCS Advisory Group. The role of healthcare professionals within critical care services. London, UK.
Österreichische Apothekerkammer: Apotheke in Zahlen (2020) Available at https://www.apothekerkammer.at/infothek/zahlen-daten-fakten/apotheke-in-zahlen-2020
Österreichische Apothekerkammer: Weiterbildungsordnung - Krankenhausfachapotheker (KhFA-WbO 2015 idgF) Available at https://www.apothekerkammer.at/infothek/rechtliche-hintergruende/apothekerkammer-und-apothekerberufsrecht/weiterbildungsordnung-krankenhausfachapotheker-khfa-wbo-2015
Newsome A, Murray B, Smith S et al. (2021) Optimization of critical care pharmacy clinical services: A gap analysis approach. Am J Health Syst Pharm. 78(22):2077-2085.
Quintens C, Peetermans W, Lagrou K et al. (2021) The effectiveness of Check of Medication Appropriateness for antimicrobial stewardship: an interrupted time series analysis. J Antimicrob Chemother. 77(1):259-267.
Quintens C, Peetermans W, Van der Linden L et al. (2022) End-users feedback and perceptions associated with the implementation of a clinical-rule based Check of Medication Appropriateness service. BMC Med Inform Decis Mak. 22(1):177.
Quintens C, Verhamme P, Vanassche T et al. (2022) Improving appropriate use of anticoagulants in hospitalised patients: A pharmacist-led Check of Medication Appropriateness intervention. Br J Clin Pharmacol. 88(6):2959-2968.
Somers A, Spinewine I, Spriet S et al. (2019) Development of clinical pharmacy in Belgian hospitals through pilot projects funded by the government. Acta Clinica Belgica. 74(2):75-81
Stemer G, Laml-Wallner G, Kuegler I et al. (2012) Comprehensive evaluation of clinical pharmacists’ interventions in a large Austrian tertiary care hospital, European Journal of Hospital Pharmacy: Science and Practice. 19:529–534.
The Fundamentals of Critical Care Clinical Pharmacy (2024) Available at https://sites.google.com/view/fundamentalsofcriticalcare24
Van Daele R, Wauters J, Elkayal O et al. (2022) Liposomal amphotericin B exposure in critically ill patients: a prospective pharmacokinetic study. Med Mycol. 60(10):myac074.
Van De Sijpe G, Quintens, C, Walgraeve K et al. (2022) Overall performance of a drug–drug interaction clinical decision support system: quantitative evaluation and end-user survey. BMC Med Inform Decis Mak. 22:48
Wallenburg E, Ter Heine R, de Lange D et al. (2021) High unbound flucloxacillin fraction in critically ill patients. J Antimicrob Chemother. 76(12):3220-3228.
Waydhas C, Riessen R, Markewitz A et al. (2022) Empfehlung zur Struktur und Ausstattung von Intensivstationen 2022. Divi Zeitschrift.
Waydhas C, Riessen R, Markewitz A et al. (2023) DIVI-Empfehlung zur Struktur und Ausstattung von Intensivstationen 2022 (Erwachsene) [DIVI-Recommendations on the infrastructure of adult intensive care units]. Med Klin Intensivmed Notfmed. 18(7):564-575.
ZorgInstituut, Kwaliteitsstandaard Organisatie van Intensive Care (2016) Available at https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/07/07/kwaliteitsstandaard-organisatie-van-intensive-care












