ICU Management & Practice, Volume 23 - Issue 3, 2023
While intravenous fluids have traditionally been a routine treatment for most critically ill patients, many severe pathologies now suggest a preference for conservative fluid therapy over liberal fluid administration.
Introduction
Intravenous fluid resuscitation began in 1832 during the cholera pandemic, improving intravascular volume and electrolyte recovery in patients with severe hypovolaemic shock secondary to dehydration from severe diarrhoea. In critically ill patients, the aim of intravenous fluid therapy is to increase cardiac output to improve macro and microcirculation and the delivery of oxygen to tissues (DO2). However, volume status is only one of the determinants for DO2, and paradoxically, there is dilution of oxygen with fluid overload, in addition to multiple adverse effects (Pérez-Nieto et al. 2021; Messina et al. 2022). Therefore, it is important to determine to whom, when, and how much intravenous fluids to administer, as their routine and excessive use is associated with poor outcomes, such as increased mortality, mechanical ventilation (MV) days, and acute kidney injury (AKI) (Pérez-Nieto et al. 2021).
In this review, we will discuss the aspects of intravenous fluid therapy in different scenarios with the aim of promoting rational use. Doing so involves reducing the use of unnecessary resources, resulting in lower expenditure on crystalloid fluids and lower costs due to their possible complications.
Fluids in Sepsis and Septic Shock
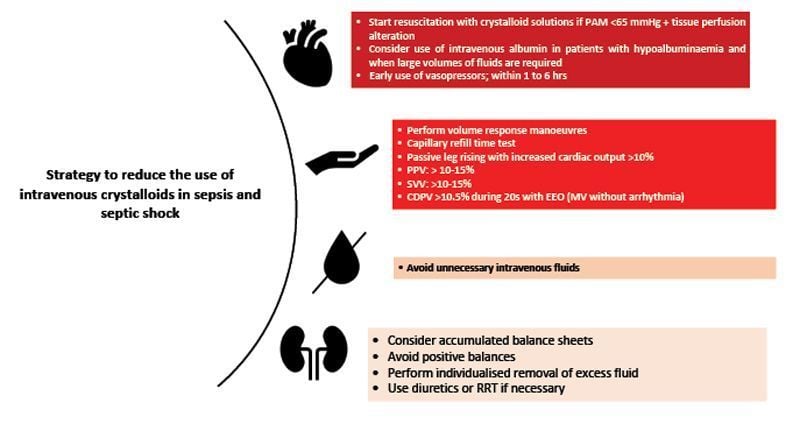
The Surviving Sepsis Campaign recommendation for the initial management of septic shock is to administer at least 30 ml/kg of intravenous fluids during the first three hours of resuscitation; however, the quality of evidence supporting this practice is low (Dellinger et al. 2021). Adequate fluid response is commonly defined as an increase in preload induced by a fluid infusion that generates an increase in stroke volume (SV) and hence cardiac output (CO) by more than 10-15%, and one of the major limitations is the lack of continuous CO measuring devices for all critically ill patients (Pérez-Nieto et al. 2019).
Initially, it has been shown that only about 50% of critically ill patients will be adequately responsive to intravenous fluid therapy, and in those sepsis patients who are initially fluid responsive, the probability of a beneficial response decreases rapidly to less than 5% within the first eight hours after resuscitation onset, according to a post-hoc analysis of the ANDROMEDA SHOCK study (Kattan et al. 2020). Patients who do not tolerate fluids adequately may develop congestion and overload with any extra amount of fluids administered (Perez-Nieto et al. 2021).
In recent years, important studies on fluid therapy in sepsis have been conducted. The randomised controlled CLOVERS trial compared a restrictive fluid resuscitation strategy (500 to 2,300 ml) with concomitant use of vasopressors versus a liberal fluid strategy (2,000 to 4,500 ml) before initiating vasopressors. A lower total fluid administration during the first 24 hours was demonstrated in the restrictive group, with no differences in mortality at 90 days. Therefore, higher IV fluid intake was not associated with better outcomes but with increased use of crystalloid solutions. A cost analysis could be suggested to evaluate the economic impact of liberal practice.
Instead of initiating IV fluid resuscitation, early norepinephrine infusion to achieve a mean arterial pressure (MAP) >65 mmHg may be associated with better outcomes when compared to delayed initiation of the vasopressor, including increased survival and less IV fluid input (Colon et al. 2020; Rui Shi et al. 2020).
In terms of fluid preference, despite the theoretical benefits of using balanced solutions (PlasmaLyte, Ringer lactate, Hartmann) that may include lower incidence of hyperchloraemia and metabolic acidosis, multiple studies in the last years have failed to demonstrate superiority in important outcomes such as mortality or development of AKI when comparing 0.9% sodium chloride solution with different types of balanced solutions (Hammond et al. 2020; Monnet et al. 2023), and the cost of the latter is commonly higher (Taylor et al. 2021).
Another circumstance to consider is the source of infection. For example, a patient with abdominal sepsis with nausea, vomiting, and poor fluid intake prior to admission is more likely to respond to IV fluids, while a patient with severe viral pneumonia is less likely to benefit from them and is more susceptible to local damage.
In summary, the benefit of administering large amounts of intravenous fluids in patients with sepsis and septic shock has been questioned in the last decade, and the recommendation for this strategy has lost strength. We suggest that the clinical benefit of fluid therapy in each patient should be weighed, considering their comorbidities, haemodynamic status, and source of infection.
Fluids in Acute Respiratory Distress Syndrome
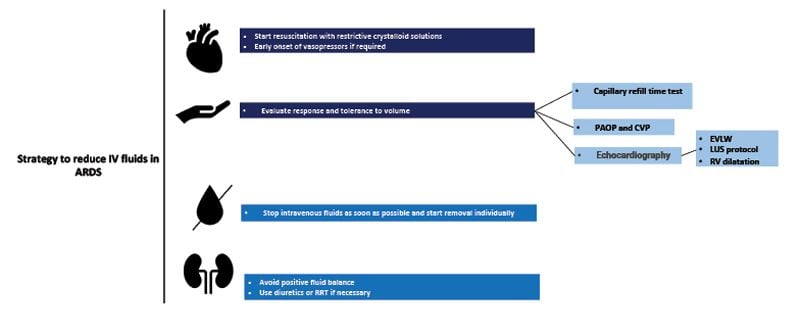
An important pathophysiological characteristic in the development of acute respiratory distress syndrome (ARDS) is an increase in the permeability of the alveolar-capillary membrane, allowing intravascular fluid to leak into the interstitial and alveolar space, causing pulmonary oedema and gas exchange impairment (Vignon et al. 2020). A common problem in these patients is that several causes of ARDS are accompanied by hypotension and shock (e.g., severe pneumonia, septic shock, severe pancreatitis, thoracic trauma, etc.), which implies the use of large amounts of intravenous fluids in some cases to restore intravascular volume, but with the secondary effect of increasing extravascular lung water (EVLW) and worsening hypoxaemia.
Improved lung function and decreased days on mechanical ventilation and ICU have been shown with a conservative fluid therapy approach in patients with ARDS, allowing the use of furosemide versus a liberal therapy. There was no difference in mortality or development of organ failure in the conservative group. There is a positive correlation between cumulative fluid balance and mortality and ICU stay in patients with ARDS (Van Mourik et al. 2019). The current recommendation for fluid management in ARDS is to provide conservative therapy (Griffith et al. 2019).
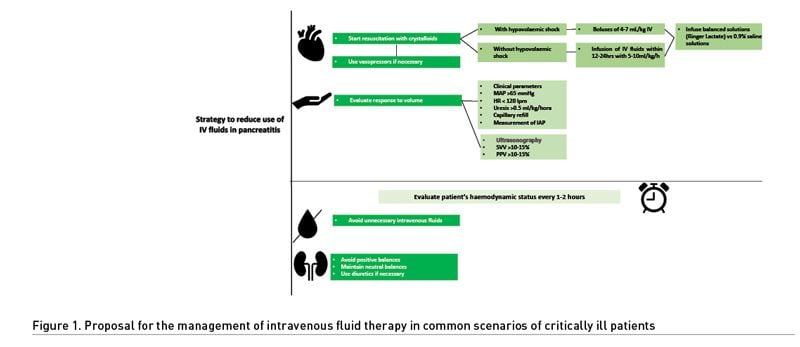
Fluids in Acute Pancreatitis
Acute pancreatitis is characterised by a significant release of proinflammatory cytokines locally and then systemically, which causes microcirculatory damage due to endothelial injury. Initially, it presents with increased CO, but during its progression, hypotension and shock may develop due to cytokine-mediated vasodilation (Crosignani et al. 2022). Various factors can contribute to fluid loss in pancreatitis, including vomiting, feeding difficulty, abdominal pain, systemic inflammation, and fever, which are associated with increased vascular permeability and outflow of intravascular fluid into the interstitial spaces and serosa (pleura, peritoneum), leading to distributive shock with a hypovolaemic component (Crosignani et al. 2022). This circulatory disturbance contributes to tissue hypoperfusion and favours organ failure (Sureka et al. 2016).
Researchers postulated two decades ago that aggressive intravenous fluid therapy could improve pancreatic perfusion and prevent necrosis in patients with mild and moderate pancreatitis. However, this theory could not be proven, and considering the latest studies, we have strong findings against this type of management.
Ten years ago, management guidelines for acute pancreatitis recommended aggressive intravenous fluid therapy at a dose of 250 to 500 mL of crystalloid solution per hour for the first 12 to 24 hours (Tenner et al. 2013). More recent recommendations suggest using fluid therapy and monitoring patients for signs of fluid overload without specifying the infusion dose during the first 72 hours. Emphasis is placed on replacing volume lost due to intolerance of the oral route and second- or third-space leakage.
However, in patients with pancreatitis, excessive fluid intake can increase the risk of elevated intra-abdominal pressure (IAP) and cause abdominal compartment syndrome, which can worsen cardiovascular, renal, intestinal, and pulmonary dysfunction and increase the risk of mortality (DeLaet et al. 2020). The most recent proposal for the resuscitation of patients with pancreatitis is goal-guided resuscitation, and the use of ultrasonography to identify evidence of venous congestion may be useful (Argaiz et al. 2021).
A recently published randomised controlled trial evaluating a conservative fluid strategy compared to aggressive fluid therapy in the first hours of care for patients with acute pancreatitis could not demonstrate benefit to prevent the progression of disease severity with aggressive fluid intake; however, it did demonstrate a greater quantity of intravenous solutions administered and an increased incidence of rales (de-Madaria et al. 2022).
Other studies report similar findings. A systematic review of randomised controlled trials with meta-analysis found an increase in mortality and complications caused by fluid overload in patients with acute pancreatitis who were managed with aggressive fluid therapy, regardless of its degree of severity, compared to conservative fluid therapy (Li et al. 2023).
Fluids in Diabetic Ketoacidosis
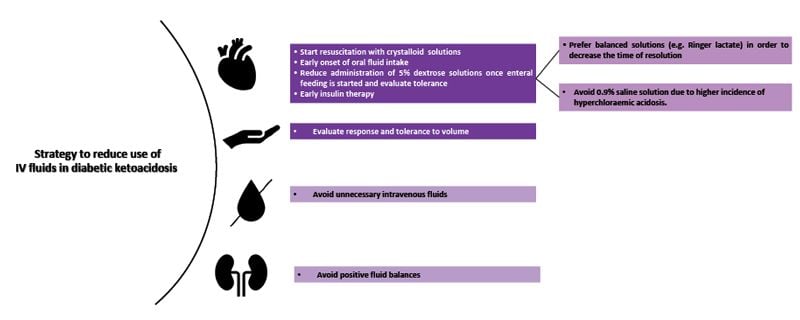
Diabetic ketoacidosis (DKA) is a serious complication of diabetes caused by an increase in serum ketones as a way of obtaining energy during acute stress and a significant decrease in insulin levels, either pancreatic or due to inappropriate treatment, culminating in metabolic acidosis, sustained hyperglycaemia, dehydration from osmotic diuresis, nausea and vomiting. Guideline-recommended treatment includes the aggressive infusion of intravenous fluids, electrolyte replacement, and insulin administration. The current recommendation is to administer an infusion of 500 mL of 0.9% sodium chloride solution to achieve a systolic blood pressure >90 mmHg, followed by 1,000 mL over 1 hour, then 1,000 mL over 2 hours, and finally 1,000 mL over 4 hours, with concurrent potassium replacement. This is based on the replacement of lost fluids, estimated at 100 ml/kg, a completely arbitrary measure. It's worth mentioning that no studies support this recommendation, despite the recommendation being universally approved (Dhatariya et al. 2022). We must remember that patients with DKA are not exempt from complications associated with fluid overload, such as pulmonary oedema (Sprung et al. 1980).
A systematic review of randomised controlled trials on patients younger than 18 years with DKA, comparing liberal and rapid infusions of IV fluids to conservative and slow therapy, found no clear benefit of one therapy over the other nor an increased incidence of major adverse effects like cerebral or pulmonary oedema. However, the liberal group showed a higher incidence of hyperchloraemic acidosis and hypocalcaemia (Long and Gottlieb 2022). No similar studies have been conducted on adult patients.
Regarding the type of solution administered, balanced solutions generate greater benefits for patients with DKA when compared to sodium chloride solution. The SKOPE-DKA study demonstrated a decrease in the resolution time of ketoacidosis symptoms without presenting a significant difference in complications when balanced solutions were compared to saline solution (Ramana et al. 2021). A recent systematic review of randomised controlled trials comparing saline with balanced crystalloids demonstrated a shorter time to resolution of DKA, fewer length of hospital stays, lower serum chloride levels, and higher bicarbonate levels (Alghamdi et al. 2022).
Fluids in Acute Kidney Injury
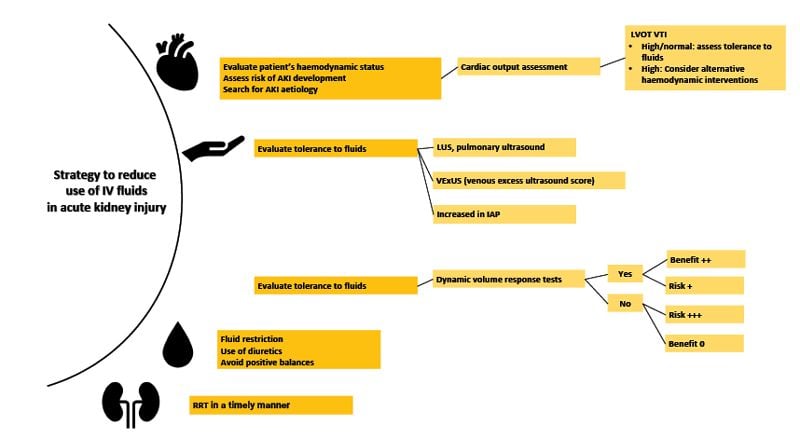
Acute kidney injury (AKI) is a common occurrence in critically ill patients and is an independent factor in mortality, particularly when presenting as oliguria or anuria. According to the multinational AKI EPI study, 57.3% of ICU patients will experience AKI symptoms during their stay, with 23.5% of them requiring renal replacement therapy (RRT). The main causes include sepsis, hypovolaemia, the use of nephrotoxic drugs, cardiogenic shock, hepatorenal syndrome, and obstructive urinary tract problems (Hoste et al. 2015).
Pathophysiologically, when AKI is caused by absolute or relative hypovolaemia, it may improve with the administration of oral, enteral, or IV fluids. However, the idea that AKI from other causes can be treated with intravenous fluid infusion has led to erroneous practices and worsening prognosis for these patients, particularly those who are unresponsive or unable to tolerate them. In addition, fluid overload can worsen or cause AKI by the following mechanisms (Mårtensson and Bellomo 2015):
| a) | Activation of tubuloglomerular feedback: the infusion of saline solutions and subsequent administration of large amounts of chlorine can activate the macula densa, which secretes vasoconstrictor substances from the afferent arteriole. This can decrease renal blood flow and, subsequently, the glomerular filtration rate. |
| b) | Increased intravascular oncotic pressure: This is generated by the administration of osmotically active substances. |
| c) | Osmotic nephrosis: This condition is characterised by vacuolisation and oedema of the proximal tubular cells. The most related causal substances are mannitol and hydroxyethyl starch (a synthetic colloid currently not recommended). |
| d) | Oedema of the renal parenchyma: This generates an increase in the distance needed for the diffusion of oxygen in the nephron, promoting renal ischaemia. |
Conclusion
Studies have shown that large amounts of intravenous solutions administered to critically ill patients are of no benefit and are commonly associated with adverse effects, such as AKI, more days on mechanical ventilation, longer stays in the ICU and hospitalisation, and increased mortality. However, patients with hypovolaemic shock and severe dehydration may benefit from intravenous fluids.
In addition, the acquisition and administration of large quantities of solutions of different types have an economic and ecological impact. The approximate cost per 100 mL of 0.9% sodium chloride solution is £0.47 ($0.6 USD), while the cost of balanced solutions is higher, with PlasmaLyte being the most expensive, at £2.25 to £3 ($3-$4 USD) per 100 mL (Taylor et al. 2021).
A conservative approach to intravenous fluids should be adopted for patients with ARDS, acute pancreatitis, and AKI. It should also be carefully considered in septic shock and other critical illnesses, not only to improve prognosis but also to reduce consumption and spending due to unnecessary interventions. In Figure 1, we present a proposal for the management of intravenous fluid therapy in common scenarios of critically ill patients.
Conflict of Interest
None.
References:
Alghamdi NA, Major P, Chaudhuri D et al. (2022) Saline Compared to Balanced Crystalloid in Patients With Diabetic Ketoacidosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Explor. 4(1):e0613.
Argaiz ER, de Moraes AG (2021) Acute pancreatitis. Lancet. 397(10271):279.
Berthelsen RE, Perner A, Jensen AK et al. (2018) Fluid accumulation during acute kidney injury in the intensive care unit. Acta Anaesthesiol Scand. 62(6):780-790.
Besen BAMP, Boer W, Honore PM (2021) Fluid management in diabetic ketoacidosis: new tricks for old dogs? Intensive Care Med. (11):1312-1314.
Colon Hidalgo D, Patel J, Masic D et al. (2020) Delayed vasopressor initiation is associated with increased mortality in patients with septic shock. J Crit Care. 55:145-148.
Crosignani A, Spina S, Marrazzo F et al. (2022) Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: a narrative review. Ann Intensive Care. 12(1):98.
Cuéllar-Monterrubio JE, Monreal-Robles R, González-Moreno EI et al. (2020) Nonaggressive Versus Aggressive Intravenous Fluid Therapy in Acute Pancreatitis With More Than 24 Hours From Disease Onset: A Randomized Controlled Trial. Pancreas. 49(4):579-583.
De Laet IE, Malbrain MLNG, De Waele JJ (2020) A Clinician’s Guide to Management of Intra-abdominal Hypertension and Abdominal Compartment Syndrome in Critically Ill Patients. Crit Care. 24, 97.
Dellinger PR, Rhodes A, Evans L et al. (2023) Surviving Sepsis Campaign. Critical Care Medicine. 51(4):431-444.
de-Madaria E, Buxbaum JL, Maisonneuve P et al. (2022) Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N Engl J Med. 387(11):989-1000.
Dhatariya KK; Joint British Diabetes Societies for Inpatient Care (2022) The management of diabetic ketoacidosis in adults-An updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med. 39(6):e14788.
Gardner TB (2022) Fluid Resuscitation in Acute Pancreatitis - Going over the waterfall. N Engl J Med. 387(11):1038-1039.
Geng L, Tian X, Gao Z et al. (2023) Different Concentrations of Albumin Versus Crystalloid in Patients with Sepsis and Septic Shock: A Meta-Analysis of Randomized Clinical Trials. J Intensive Care Med. 8850666231170778.
Gershkovich B, English SW, Doyle MA et al. (2019) Choice of crystalloid fluid in the treatment of hyperglycemic emergencies: a systematic review protocol. Syst Rev. 8(1):228.
Grams ME, Estrella MM, Coresh J et al. (2011) Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 6(5):966-73.
Griffiths MJD, McAuley DF, Perkins GD et al. (2019) Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 6(1):e000420.
Hammond DA, Lam SW, Rech MA et al. (2019) Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. Ann Pharmacother. 54(1):5-13.
Hoste EA, Bagshaw SM, Bellomo R et al. (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41(8):1411-23.
Kattan E, Ospina-Tascón GA, Teboul JL et al. (2020) Systematic assessment of fluid responsiveness during early septic shock resuscitation: secondary analysis of the ANDROMEDA-SHOCK trial. Crit Care. 24(1):23.
Lewis SR, Pritchard MW, Evans DJ et al. (2018) Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 8(8):CD000567.
Li XW, Wang CH, Dai JW et al. (2023) Comparison of clinical outcomes between aggressive and non-aggressive intravenous hydration for acute pancreatitis: a systematic review and meta-analysis. Crit Care. 27(1):122.
Long B, Gottlieb M (2022) Liberal versus conservative intravenous fluid administration in pediatric diabetic ketoacidosis. Academic Emergency Medicine.
Mårtensson J, Bellomo R (2015) Are all fluids bad for the kidney? Curr Opin Crit Care. 21(4):292-301.
Matthay MA, Zemans RL, Zimmerman GA et al. (2019) Acute respiratory distress syndrome. Nat Rev Dis Primers. 5(1):18.
Mehta RL, Pascual MT, Soroko S et al. (2004) Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 66(4):1613-21.
Messina A, Bakker J, Chew M et al. (2022) Pathophysiology of fluid administration in critically ill patients. Intensive Care Medicine Experimental. 10, 46.
Monnet X, Lai C, Teboul JL (2023) How I personalize fluid therapy in septic shock? Crit Care. 27(1):123.
Mustafa OG, Haq M, Dashora U et al. (2023) Management of Hyperosmolar Hyperglycaemic State (HHS) in Adults: An updated guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Diabet Med. 40(3):e15005.
National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network; Shapiro NI, Douglas IS, Brower RG et al. (2023) Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 388(6):499-510.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann HP, Wheeler AP, Bernard GR et al. (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 354(24):2564-75.
Ostermann M, Liu K, Kashani K (2019) Fluid Management in Acute Kidney Injury. Chest. 156(3):594-603.
Pérez Nieto OR, Wong A, Lopez Fermin J et al. (2021) Aiming for zero fluid accumulation: First, do no harm. Anaesthesiol Intensive Ther. 53(2):162-178.
Pérez Nieto OR, Sánchez-Díaz JS, Solórzano-Guerra A, Márquez-Rosales E (2019) Fluidoterapia intravenosa guiada por metas. Med Int Méx. 35(2):235-250.
Rabindranath K, Adams J, Macleod AM, Muirhead N (2007) Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. (3):CD003773.
Ramanan M, Attokaran A, Murray L et al. (2021) Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): a cluster, crossover, randomized, controlled trial. Intensive Care Med. 47(11):1248-1257.
Salahuddin N, Sammani M, Hamdan A et al. (2017) Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: results of a cohort study. BMC Nephrol. 18(1):45.
Shi R, Hamzaoui O, De Vita N et al. (2020) Vasopressors in septic shock: which, when, and how much? Ann Transl Med. 8(12):794.
Sprung CL, Rackow EC, Fein IA (1980) Pulmonary edema; a complication of diabetic ketoacidosis. Chest. 77(5):687-688.
Sureka B, Bansal K, Patidar Y, Arora A (2016) Imaging lexicon for acute pancreatitis: 2012 Atlanta Classification revisited. Gastroenterol Rep (Oxf). 4(1):16-23.
Taylor C, Yang L, Finfer S et al. (2021) An international comparison of the cost of fluid resuscitation therapies. Aust Crit Care. 34(1):23-32.
Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology (2013) American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 108(9):1400-15; 1416.
Tomita H, Ito U, Tone O et al. (1994) High colloid oncotic therapy for contusional brain edema. Acta Neurochir Suppl (Wien). 60:547-9.
Van Mourik N, Metske HA, Hofstra JJ et al. (2019) Cumulative fluid balance predicts mortality and increases time on mechanical ventilation in ARDS patients: An observational cohort study. PLoS One. 14(10):e0224563.
Vignon P, Evrard B, Asfar P et al. (2020) Fluid administration and monitoring in ARDS: which management? Intensive Care Med. 46(12):2252-2264.
Wu F, She D, Ao Q et al. (2022) Aggressive intravenous hydration protocol of Lactated Ringer’s solution benefits patients with mild acute pancreatitis: A meta-analysis of 5 randomized controlled trials. Front Med. 9:966824.
Yerram P, Karuparthi PR, Misra M (2010) Fluid overload and acute kidney injury. Hemodial Int. 14(4):348-54.












