1. Sepsis: the Need for Speed
Sepsis is a life-threatening form of organ dysfunction caused by a dysregulated host response to infection1. This extremely heterogeneous and dynamic syndrome is the result of complex interactions between invading microorganisms and the host’s immune system. Disruption of the host’s ability to maintain immune homeostasis and to mount an effective response to the infectious agent can lead to hypoperfusion, acute alteration of mental status, organ dysfunction, and death. Sepsis is a major healthcare burden, with an estimated 48.9 million cases occurring in 2017; it claims more than 11 million lives per year2
Sepsis requires prompt and tailored treatment; any delay in its diagnosis increases the risks of mortality and progression toward septic shock, which is associated with hypotension and perfusion abnormalities. Each hour of delay in the initiation of suitable antibiotic therapy was shown to decrease the survival of patients with septic shock by 7.6%3. Often a silent disease, sepsis seldom presents with clear signs and symptoms that would enable physicians to diagnose it early, until sudden clinical degradation occurs, with adverse and often fatal outcomes. The well-accepted concept that early action limits the damage resulting from sepsis forms the basis of early goal-directed therapy, which has been demonstrated to reduce in-hospital mortality after the implementation of a sepsis bundle4,5. Since the clinical complications of infection are reflected first by changes at the molecular and cellular levels, and later by the appearance of clinically overt signs, the timely measurement of sensitive and specific biomarkers can provide a unique window of opportunity for the timely initiation of adequate treatment. In addition to requiring early management, the specific identification of sepsis and differentiation from non-infectious inflammation is important to avoid the over diagnosis and futile administration of antibiotics, responsible for the selection of antibiotic resistant bacteria. Antimicrobial resistance (AMR) poses a major threat to human health around the world. In 2019, 4.95 million (3·62–6·57) deaths were associated with bacterial AMR and 1,27 million (95% UI 0·911–1·71) deaths were attributed to AMR6.
Therefore, the availability of an early and accurate sepsis biomarker at the patient’s bedside is key to transforming sepsis care by enabling faster access to treatment, tailoring treatments, decreasing mortality and by lowering sepsis-related healthcare costs.
 | The Economic Impact of Early Sepsis Diagnosis Sepsis is the most expensive hospital condition to treat and the leading cause of in-hospital death due to a challenging diagnosis. An early recognition of sepsis can trigger early treatment and contribute to significant reductions in ICU lengths of stay, severity of sepsis, readmission rates and long-term consequences of sepsis and ultimately, reductions in the total cost per case2,7. An analysis of US Medicare claims found that costs associated with sepsis reached $22.4 billion in 20188. In a model study conducted in the US, PSP-guided sepsis care reduced the expected per-person cost of treatment by 8% compared to the standard of care, corresponding to $1,847 savings per sepsis case. Extrapolation to the 1.7 million sepsis cases per year, this suggests that the nationwide savings may reach up to $3.1 billion9. Although costs vary among countries and healthcare institutions, it is widely accepted that any measure taken to improve the recognition and treatment of sepsis will reduce the economic burden and devastating impact on patients. |
2. Pancreatic Stone Protein
Pancreatic stone protein (PSP) is produced mainly by the pancreas, small intestine, and stomach10, and increased PSP blood concentrations are associated with the development of sepsis11,12. Structurally, PSP is a globular 16-kilodalton polypeptide belonging to the C-type lectin family of proteins, which are calcium-dependent glycan-binding proteins that play essential roles in homeostasis, innate immunity, and the inflammatory response 13. These structural traits link PSP to the host response to infection. In vitro, PSP activates polymorphonuclear neutrophil granulocytes (PMNs) by regulating the shedding of L-selectin (CD62L) and the membrane expression of ß2-integrin (CD11b) 10,14, two well-known markers of PMN activation that mediate adherence to endothelial cells in vessel walls, thereby moderating their recruitment and trafficking to the infection site.
PSP is detectable at low levels in the bloodstream in healthy neonates, children, and adults. In a cohort of healthy adults, PSP levels ranged from 23 to 74 ng/ml 15. The normal range, defined by the 5th and 95th percentiles, was 27–61 ng/ml, and the median concentration was 42 ng/ml. No significant difference by gender, race/ethnicity, or age ≥ or < 60 years was observed (Table 1) 15. Using an investigational-use-only PSP microtiter-plate ELISA, Schlapbach and colleagues16 showed that PSP values increase in an age-dependent manner from birth through childhood and remain constant during adulthood.

Table 1. Normal PSP values in healthy adults, partitioned by gender, age group, and race/ethnicity. P < 0.05 was defined as significant. ND, not determined; NS, not significant; PSP, pancreatic stone protein.
PSP levels can be measured rapidly (in as little as 7.5 minutes) at the point of care from a single drop of whole blood on Abionic’s nanofluidic-based platform, the abioSCOPE®17. This platform enables the close monitoring of blood PSP concentrations associated with the early development of sepsis, providing immediate, actionable results (Figure 1).

Figure 1. (A) Point-of-care abioSCOPE® device and kits and (B) schematized trajectories of PSP in patients who develop nosocomial sepsis (dashed red line) or not (solid black line). An increasing PSP in the days preceding the clinical diagnosis of sepsis offers a unique window of opportunity for Clinicians to perform additional diagnostic investigations and to start therapeutic interventions aimed at mitigating the impacts of sepsis. See earlier - Act better.
The PSP test performed on the abioSCOPE® device is the first-ever commercially available CE-marked in vitro diagnostic test for the measurement of this protein. Abionic SA is therefore setting the standard for PSP quantification in whole blood. Before the commercialization of this test, various research-use-only techniques were used for PSP measurement. Given differences in assay design, technology, and calibration strategy, published absolute PSP values and clinical cutoffs may differ from those obtained with the abioSCOPE®; thus, caution should be taken when interpreting concentrations.
 | Did you know? Discovered in the early 1980s, PSP was first independently named “lithostatine” and “regenerating protein 1 (REG1)” by separate research groups working on diabetes and pancreatitis, respectively 18. It was initially hypothesized to be an inhibitor of pancreatic stone formation, but a group led by Prof. Rolf Graf in Zurich, Switzerland, made an essential, albeit somewhat serendipitous, discovery. In a cohort of patients with polytrauma, they observed a constant and specific increase in the PSP concentrations of patients who developed sepsis. In contrast to other canonical markers of sepsis, PSP levels remained stable in patients with trauma-related hyperinflammation who did not develop sepsis, 19. Subsequent clinical investigations, especially in the last 10+ years, have established the role of PSP as a mediator of septic events, and in particular as an early biomarker for the identification of sepsis 18,20. |
3. Use of the PSP Biomarker for the Diagnosis of Sepsis and Nosocomial Sepsis in Adults
PSP can differentiate, more accurately than CRP and PCT, infection from sepsis and an inflammatory state from sepsis in intensive care patients including severely burns, in postoperative patients including cardiac surgery, in trauma patients and also in patients with inhalation lung injury.
In addition, PSP outperforms classical biomarkers of sepsis thanks to an earlier relative increase before clinical diagnosis 21
In addition, a constantly low PSP value is a strong indicator to rule out sepsis (with high negative predictive value) in patients presenting a sepsis-like clinical picture. In such cases, no additional microbiological investigation or other sepsis-focused procedures are recommended. A low, stable PSP level also supports the decision to not administer or initiate unnecessary empiric antimicrobial treatment (Figure 13).
3.1 Identification of Sepsis at ICU Admission
Rapid, reliable, and early biomarkers that help physicians distinguish patients with sepsis from those with non-infective systemic inflammatory response syndrome (SIRS) are urgently needed 22. Biomarkers have always played essential roles in the diagnosis of sepsis, but canonical markers such as C-reactive protein (CRP) and procalcitonin (PCT) have intrinsic limitations. In a landmark study of commonly used and novel sepsis biomarkers, PSP showed the greatest accuracy in the differentiation of sepsis from non-infectious SIRS at the time of admission to high-dependency units and ICUs; its diagnostic accuracy for sepsis in 162 unselected critically ill patients was 93% (Table 2) 23. The PSP concentration correlates well with illness severity, enabling the personalized clinical management of patients in the ICU (Figure 2).
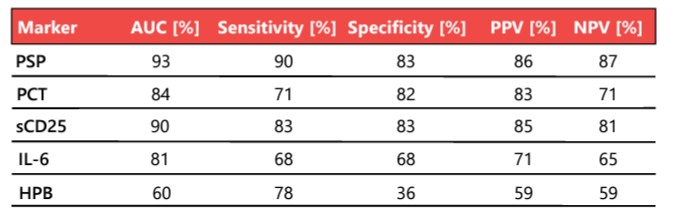
Table 2. Biomarker performance in the distinction of sepsis (n = 87) from SIRS without infective etiology (n = 75). AUC, area under the curve; HPB, heparin binding protein; IL-6, interleukin 6; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; PSP, pancreatic stone protein; sCD25, soluble CD25. Adapted from 23.

Figure 2. Biomarker levels in patients with SIRS and sepsis at the time of admission to high-dependency units or ICUs. Boxes show medians with interquartile ranges, and whiskers show the 5th and 95th percentiles. The dotted horizontal line represents the cutoff for the distinction of sepsis from non-infective SIRS, obtained by receiver operating characteristic analysis. * P < 0.05, ** P < 0.01, *** P < 0.001, independent-samples t test. SIRS, systemic inflammatory response syndrome. Adapted from 23
3.2 Daily Monitoring and Early Diagnosis of Nosocomial Sepsis
3.2.1. In the ICU
Nosocomial sepsis occurs frequently in critically ill adults and is a substantial ICU burden associated with longer hospital stays and increased mortality and costs 24. In addition to the diagnosis of sepsis at the time of ICU admission, the early identification of nosocomial sepsis acquired during hospitalization is of the utmost importance to mitigate its impact. A primary advantage of PSP over canonical sepsis biomarkers, is that its concentration increases early, up to 72 hours in advance, in the course of sepsis development 11,12. This early increase is exemplified well in the typical case of a 71-year-old patient with traumatic brain injury, in whom an increase in the PSP concentration early in sepsis development was observed, opening a window of opportunity for timely, targeted clinical management (Box 3). The association of a continuous increase in the PSP concentration with the development of nosocomial sepsis in critically ill adult patients was demonstrated initially in patients with severe burns (Section 3.2.2.) and patients admitted to the ICU with polytrauma (Section 3.2.4.). It was confirmed in a large multicentric study of critically ill adults, published in Critical Care in 2021; a linear mixed-effects model revealed a much stronger association of biomarker increase in the days preceding sepsis with subsequent sepsis development for PSP than for CRP and PCT (Figure 3)11. At the population level, PSP values in patients developing nosocomial sepsis peaked on day 6, whereas the median timepoint of nosocomial sepsis diagnosis was day 7; PSP levels remained relatively stable in patients without nosocomial sepsis (Figure 4) 11. This multicentric study also showed that the combination of PSP and CRP had the best diagnostic accuracy (AUC 0.79 95%CI 0.72-0.86) the day of sepsis compared to procalcitonin (AUC 0.75 95%CI 0.68-0.82), either biomarker alone or other combinations of biomarkers.

Figure 3. The average daily PSP concentration with SEM preceding the diagnosis of nosocomial sepsis (day 0; red line) or ICU discharge for patients who did not develop sepsis (black line). PSP, pancreatic stone protein; SEM, standard error of the mean. Adapted from 11.
 | A Case Report In a case issued from the AB-PSP-001 clinical study11, a 71-year-old male patient was taken to hospital for a traumatic brain injury, which necessitated immediate ICU admission with invasive mechanical ventilation. On day 2, the patient underwent external ventricular drainage. On day 10, a pulmonary aspiration identified a Corynebacterium propinquum infection and on day 12 a bacteremia caused by Fusobacterium nucleatum was found. The patient had a severe mesenteric infarction on day 12 and died. The patient’s PSP, CRP, and PCT levels were relatively low on admission. The CRP level was elevated from day 2 onward, peaking at 165 mg/l on day 4. The PCT level was <0.2 ng/ml through day 10, then increased to 0.7 ng/ml on day 11 and 2.4 ng/ml on day 12. The PSP level remained stable and low until day 7, when it began to increase, eventually reaching clinical elevation. The continuous increase in the PSP concentration between days 7 and 10 was associated with the development of bacteremia. 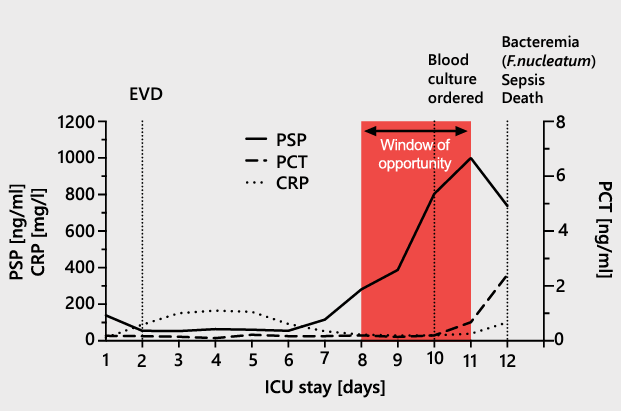 |
Figure 4. PSP trajectories in a cohort if critically ill adults who did (red) or did not (black) develop sepsis. In this cohort, the median time to sepsis diagnosis was 6 days. Data are median with SEM. Unpublished data from AB-PSP-001 (NCT: 03474809). PSP, pancreatic stone protein; SEM, standard error of the mean.
3.2.2. Patients with Severe Burns
Burn victims’ state of persistent hyperinflammation, metabolic disruption, and capillary leakage often camouflages clinical signs associated with sepsis, thereby delaying the initiation of therapy. The prevalence of sepsis in these patients ranges from 8% to 43%25.
In addition, the definitions themselves can be a source of confusion. In a recent study, 4 biomarkers were evaluated for their performance to diagnose sepsis according to 3 definitions of sepsis, including: Sepsis-3, Sepsis American Burn Association (ABA) 2007 and Sepsis Zurich Burn Center. PSP had notably the highest accuracy for septic versus infected patients according to all 3 definitions in the 3 days leading up to the event (seeFigure 5)

Figure 5. ROC-curve analysis with AUC for Sepsis-3 of PSP at t = -3, -2, -1 of 0.72, 0.88 and 0.85 respectively before the event of sepsis. The sensitivity at t = -3, -2 and -1 is 0.7, 0.8 and 0.7 respectively, and specificity is 0.7, 0.81 and 0.9 respectively.
The PSP is more specific to sepsis in burn patients than PCT, interleukin (IL)-6, and CRP, and it is not altered by sterile inflammation26. In a cohort of 90 patients with severe burns (total burn surface area, > 15) PSP levels increased three-fold during the 72 hours before the clinical appearance of sepsis (Figure 6). This pattern confirms the value of PSP as an early biomarker of sepsis.

Figure 6. Evolution of the PSP level in severely burnt patients with and septic shock, sepsis, infection, or no infection, before and after the day of these events. PSP, pancreatic stone protein. Adapted from 26.
These results clearly highlight that the high predictive power and early temporal increase of PSP in septic patients can uniquely support burn specialists in their clinical decision processes 27.
Pulmonary inhalation injury in burn patients causes a systemic immune and inflammatory response that further complicates the diagnosis of sepsis, limiting the effectiveness of canonical biomarkers. Whereas other biomarkers cannot be used to differentiate patients with and without sepsis in the presence of inhalation injury, the PSP concentration increases steeply before sepsis onset regardless of such injury (Figure 7)28.



Figure 7. Daily PSP, PCT, and CRP levels in severely burnt patients with inhalation injury with and without sepsis. CRP, C-reactive protein; PCT, procalcitonin; PSP, pancreatic stone protein. * P < 0.05, ** P < 0.01. Adapted from 28
Current burn treatment protocols following ICU admission may include surgery, large wound debridement, skin grafting, and limb amputation. The tissue damage and stress caused by these interventions results in the release of pro-inflammatory cytokines, which in turn stimulate the synthesis of acute-phase proteins such as CRP, leading to extensive immune, hemodynamic, and endocrine alterations
Biomarkers such as the white blood cell count (WBC) and PCT level also change in response to thermal injury and subsequent procedures, regardless of infection 29. Only the serum PSP level demonstrates robust steadiness after initial thermal injury and subsequent procedures or surgery, making it much more reliable in this situation than the PCT and CRP levels and WBC, which increase shortly after the burn trauma and treatment (Figure 8).

Figure 8. Time course of biomarker levels in the 2 days after admission to a burn center. Geometric means with 95% confidence intervals are presented. PSP, pancreatic stone protein; PCT, procalcitonin; CRP, C-reactive protein; WBC, white blood cell count. Adapted from 29
3.2.3. Post–Cardiac Surgery Patients
Cardiac surgery procedures and cardiopulmonary bypass induce an acute inflammatory response in which routinely used inflammatory biomarkers, such as the CRP and WBC, increase, camouflaging the development of infection and sepsis. In contrast, postoperative PSP levels are not affected by extracorporeal circulation and are associated significantly with the presence of infection in this context 12.
3.2.4. Polytrauma Patients
Patients admitted to the ICU with polytrauma are subject to acute systemic inflammation. In these patients, the development of nosocomial infection or sepsis correlates well with PSP elevation in the days preceding this event, whereas it remains close to normal in those who do not experience an infectious or septic event. This PSP increase is more pronounced for sepsis than for infection (Figure 9) 19.

Figure 9. PSP levels in polytrauma patients without infection (white bars, n = 18), with local infection (light-gray bars, n = 32), and with sepsis (dark-gray bars, n = 33). The horizontal line indicates normal values of healthy volunteers. * P < 0.05 and ** P < 0.01 for comparison of patients with local infection or sepsis vs. non-infected patients. Adapted from 19.
3.2.5. Critically Ill Coronavirus Disease 2019 Patients
Certain hospitalized patients infected with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop severe complications with rapid progression toward respiratory decompensation and hemodynamic instability, necessitating immediate transfer to higher levels of care where appropriate organ-supporting therapy can be provided 30,31. These complications include septic shock and multiorgan dysfunction 32,33. Given its unique ability to predict complications of infection and unfavorable outcomes in adult patients in the ICU, PSP is an ideal marker of secondary bacterial infection in patients with severe 2019 coronavirus disease (COVID-19), which poses a diagnostic challenge 34. In a pilot study with a cohort of 127 critically ill patients with COVID-19 admitted to a single ICU, the PSP level increased rapidly and significantly 48 hours before septic shock and organ dysfunction occurred (Figure 10) 35. This level remained close to baseline in the presence of uncomplicated SARS-CoV-2 infection, supporting the specificity of PSP to secondary bacterial sepsis and/or severe disease 35.

Figure 10. PSP concentrations preceding (A) septic shock and (B) multiorgan dysfunction. PSP, pancreatic stone protein.
A case series report presented during the 38th Vicenza Course on AKI and CRRT described the role of PSP as an early marker of sepsis in 3 critically ill COVID-19 patients collected between August and October 2020. In a 55 year-old male patient, PSP anticipated the clinical suspicion of hospital acquired pneumonia by 2 days, with isolation of Klebsiella pneumonia subsequently confirmed by broncho aspiration 36.
3.3. Identification of Severe Infection in Hospitalized Patients
A systematic review and individual patient level meta-analysis published in Critical Care in 2021 revealed that PSP outperformed both CRP and PCT to diagnose severe infection in hospitalized patients 34. When not treated on time and adequately, severe infections can evolve into life-threatening sepsis. The diagnostic performances determined by area under receiver-operating characteristic (AUROC) curve analysis and 95% confidence intervals (CI) were PSP 0.81; (95%CI 0.78-0.85), CRP 0.77; (CI95% 0.73-0.80) and PCT 0.78; (95%CI 0.74-0.82). In this review 631 patients were evaluated from different populations, countries and clinical settings including critical care, high-dependency care, and emergency care, showcasing a highly heterogenous selection of patients and robust performance of PSP despite this. As for nosocomial sepsis in intensive care (3.2.1) The combination of PSP and CRP has an AUROC of 0.90 (95%CI 0.87-0.92), with a specificity of 84% (95%CI 79-90%) and a sensitivity of 81% (95%CI 77-85%).
 | A Case Report A 70-year-old male patient admitted to the ICU 35 with confirmed SARS-CoV-2 infection and interstitial pneumonia was ventilated mechanically from day 1. The patient received antibiotics daily, and the drugs were switched on days 3, 5, 8, and 12. The PSP level predicted secondary bacterial infection 48 hours before standard microbiology did and 72 hours before the onset of clinically overt septic shock. A decline in the PSP level also correlated with reduced vasopressor administration. The patient was discharged on day 21.  |
4. The Prognostic Value of PSP
Despite significant improvements in the clinical management of sepsis and septic shock (reflected in the 2016 Sepsis-3 definition and guidelines, updated regularly by the Society of Critical Care Medicine 1), these conditions remain leading causes of mortality in critically ill patients 37. PSP has shown excellent performance not only in the identification of sepsis, but also as a prognostic biomarker; in a cohort of 91 patients with peritonitis, PSP predicted postoperative mortality more accurately than CRP, PCT, IL-6, and proinflammatory cytokines 38. Moreover, the PSP concentration at the time of ICU admission for sepsis or septic shock correlated linearly with the probability of ICU mortality (Figure 11), confirming its utility for patient stratification according to the risk of unfavorable outcomes, and thereby the identification of patients who may benefit most from tailored ICU management 38. Similarly, a decrease in the PSP level following ICU admission was associated with a greater chance of survival (Figure 12) 39. Thus, PSP can be used to assist clinicians’ risk stratification and decision making in this context 40.

Figure 11. Probability of in-hospital mortality upon admission according to the concentrations of PSP (black dots), IL-6 (red dots), and PCT (orange dots). Gradients of gray correspond to quartiles of biomarker concentrations. PSP, pancreatic stone protein; IL-6, interleukin 6; PCT, procalcitonin. Adapted from 42.

Figure 12. Kaplan–Meier curves of survival of critically ill adults, according to changes in the PSP level. PSP, pancreatic stone protein. Adapted from 39.
In line with these observations, the PSP level also correlated well with the Sequential Organ Failure Assessment (SOFA) score, a clinical score that is part of the Sepsis-3 definition and correlates with in-hospital mortality, in a population of patients with ventilator-associated pneumonia 43,44.
In patients with non-infectious SIRS, the PSP level remains low independently of the SOFA score and increases proportionally with this score only in the presence of sepsis (Figure 13).

Figure 13. Relationships between the PSP level and illness severity. Boxes represent medians and interquartile ranges, and whiskers show the 5th and 95th percentiles. *P < 0.05, **P < 0.01, ***P < 0.001, pairwise group comparison. PSP, pancreatic stone protein. Adapted from 23.
 | Sepsis in Neonates and Children Sepsis is the leading cause of mortality in neonates and children 22. As in adults, the early detection, prompt administration of antibiotics, and appropriate support of dysfunctioning organs are urgently needed in these populations. Recent research has led to the establishment of PSP as a marker of multiorgan failure and mortality in neonates and children 45–49. For infants, however, ethical difficulties with the performance of large clinical trials and the difficulty of blood sampling pose challenges to research in this field. The small sample volume of capillary blood required for PSP testing with abioSCOPE® device can simplify the measurement workflow for this patient group. |
5. Implementation of PSP Measurements
Serial PSP measurements every 24 hours can aid the clinical management and early identification of sepsis in patients at risk or suspected of sepsis. This approach provides opportunities for the timely initiation of sepsis care bundles, appropriate preventive therapies and ICU monitoring of such patients.
In addition, a constantly low PSP value is a strong indicator of a patient’s stability to rule out sepsis (with high negative predictive value) in patients presenting a sepsis-like clinical picture; in such cases, no additional microbiological investigation or other sepsis-focused procedure is recommended. A low, stable PSP level also supports the decision to not administer or initiate unnecessary empiric antimicrobial treatment (Figure 14).

Figure 14. Decision tree for the interpretation of serial PSP measurement in critically ill adults with sepsis risk or suspicion. PSP, pancreatic stone protein
*Stability or improvement of the hemodynamic, respiratory and renal functions, stable metabolic state, absence or improvement of lactacte acidosis and no suspicion of infection.
As a consequence of the diverse expressions and complex interactions between the host, the pathogen and the patient's underlying clinical conditions, the absolute values and relative changes of PSP should always be evaluated in light of the entire clinical picture of the patient. Increased PSP levels may not always be caused by a sepsis event. There are few situations where high PSP values have been observed in absence of sepsis, such as acute pancreatitis45, end-stage kidney diseases or major visceral surgeries. Low PSP levels do not automatically rule out the presence of sepsis.
The PSP test performed on the abioSCOPE® device is designed for on-demand use in the ICU. Compact, robust, and intuitive to use, it is fully compatible with hospital information systems and fits seamlessly into the workflow, and can be positioned next to blood gas analyzers. When sepsis is suspected, or needs to be ruled out, an immediate access to reliable test results is essential, without the need to wait for laboratory turnaround times. The information gained from serial PSP measurements may be crucial in determining whether a patient is developing nosocomial sepsis, preceding the appearance of overt clinical signs and symptoms and guiding further diagnostic workup and therapeutic management, which may ultimately attenuate sepsis severity.
6. Key Take-Away Points
The PSP level is associated with the development of sepsis. It is a marker that aids the identification of sepsis before the appearance of overt clinical signs and symptoms. PSP measurement provides a unique opportunity for the timely initiation of tailored diagnostic workup and therapeutic intervention to mitigate the impact of sepsis.
 | A continuous increase in the PSP level is associated with the development of nosocomial sepsis. A thorough search for the source of infection, close monitoring of organ function, and consideration of local sepsis protocol initiation are recommended. |
 | A low, stable PSP level is a strong indicator of a patient’s clinical stability. No performance of additional microbiological investigation or other sepsis-focused procedure is recommended, particularly when clinical signs and laboratory data further support this finding |
 | PSP is a good biomarker of illness severity in adult patients in the ICU and high dependency care units. The PSP level at admission correlates well with the risk of mortality. PSP measurement aids the identification of patients who will benefit most from aggressive and tailored ICU management. |
All of the available evidence indicates that PSP is suited for use in:
 | Patient stratification based on disease severity and mortality risk
|
 | Anticipation of clinical degradation
|
 | Mitigation of the impact of sepsis
|
The abioSCOPE® and the daily monitoring of PSP for the identification of nosocomial sepsis is CE marked since 2020
Reference:
- Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801 (2016). ** Sepsis-3 definition
- Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395, 200–211 (2020).** Global sepsis incidence report
- Kumar, A. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*: Critical Care Medicine 34, 1589–1596 (2006).
- Ferrer, R. et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 299, 2294–2303 (2008).
- Levy, M. M. et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36, 222–231 (2010).
- Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 0, (2022.
- Paoli, C. J., Reynolds, M. A., Sinha, M., Gitlin, M. & Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit Care Med 46, 1889–1897 (2018).
- Buchman, T. G. et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012-2018. Crit Care Med 48, 276–288 (2020).
- Schneider, J., Dick, K. & Cooper, J. Pancreatic Stone Protein Point-of-Care Testing can Reduce Healthcare Expenditure in Sepsis. (Submitted).
- Reding, T. et al. The pancreas responds to remote damage and systemic stress by secretion of the pancreatic secretory proteins PSP/regI and PAP/regIII. Oncotarget 8, 30162 (2017)
- Pugin, J. et al. Serial Measurement of Pancreatic Stone Protein for the Early Detection of Sepsis in Intensive Care Unit Patients: A Prospective Multicentric Study. https://www.researchsquare.com/article/rs-150969/v1 (2021), doi:10.21203/rs.3.rs-150969/v1.** European multicentric clinical study on PSP
- Klein, H. J. et al. Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. PloS one 10, e0120276 (2015).
- Cummings, R. D. & McEver, R. P. C-Type Lectins. in Essentials of Glycobiology (eds. Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2015).
- Simon, S. I. & Green, C. E. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7, 151–185 (2005).
- Abionic. Abionic Internal Data: Normal Values of PSP.
- Schlapbach, L. J. et al. Normal values for pancreatic stone protein in different age groups. BMC Anesthesiology 15, (2015).
- Putallaz, L., Bogaard, P. V. D., Laub, P. & Rebeaud, F. Nanofluidics Drives Point-of-care Technology for on the Spot Protein Marker Analysis with Rapid Actionable Results. (2019) doi:10.35248/2157-7439.19.10.536.
- Graf, R. Pancreatic stone protein - sepsis and the riddles of the exocrine pancreas. Pancreatology 20, 301–304 (2020).
- Keel, M. et al. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes*: Critical Care Medicine 37, 1642–1648 (2009).
- Eggimann, P., Que, Y.-A. & Rebeaud, F. Measurement of pancreatic stone protein in the identification and management of sepsis. Biomark Med 13, 135–145 (2019).
- Fidalgo, P., Nora, D., Coelho, L. & Povoa, P. Pancreatic Stone Protein: Review of a New Biomarker in Sepsis. Journal of Clinical Medicine 11, 1085 (2022).
- Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Medicine 43, 304–377 (2017)
- Llewelyn, M. J. et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Critical care 17, R60 (2013). * PSP on admission to ICU and HDC.
- Vincent, J.-L. et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 323, 1478–1487 (2020).
- Mann, E. A., Baun, M. M., Meininger, J. C. & Wade, C. E. Comparison of Mortality Associated With Sepsis in the Burn, Trauma, and General Intensive Care Unit Patient: A Systematic Review of the Literature. Shock 37, 4–16 (2012).
- Klein, H. J. et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann Surg (2020) * PSP in severly burnt patients
- Niggemann, P. et al. Incidence and Time Point of Sepsis Detection as Related to Different Sepsis Definitions in Severely Burned Patients and Their Accompanying Time Course of Pro-Inflammatory Biomarkers. J Pers Med 11, 701 (2021).
- Klein, H. J. et al. Response of routine inflammatory biomarkers and novel Pancreatic Stone Protein to inhalation injury and its interference with sepsis detection in severely burned patients. Burns (2020) doi:10.1016/j.burns.2020.04.039.
- Klein, H. J. et al. Expression of Pancreatic Stone Protein is Unaffected by Trauma and Subsequent Surgery in Burn Patients. World J Surg 44, 3000–3009 (2020).
- Yuki, K., Fujiogi, M. & Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin Immunol 215, 108427 (2020).
- Jordan, R. E., Adab, P. & Cheng, K. K. Covid-19: risk factors for severe disease and death. BMJ 368, m1198 (2020).
- Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
- Li, H. et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet 395, 1517–1520 (2020).
- Prazak, J. et al. Pancreatic Stone Protein for the Diagnostic of Infection. Individual Patient Level Meta-analysis. Submitted (2021). ** Systematic review and meta-analysis on PSP
- Ventura, F. Pancreatic Stone Protein (PSP) measurement at the bed side as an early biomarker of sepsis and organ failure in critically ill COVID-19 patients. (2020).
- Morri, D. et al. Potential Role of Pancreatic Stone Protein (Psp) as Early Marker of Bacterial infection in Covid-19 Patients. 38th Vicenza Course of AKI & CRRT https://npselearning.it/img/38-vicenza/pdf/36.pdf (2021).
- Vincent, J.-L. et al. Sepsis in European intensive care units: Results of the SOAP study*. Critical Care Medicine 34, 344–353 (2006).
- Gukasjan, R., Raptis, D. A., Schulz, H.-U., Halangk, W. & Graf, R. Pancreatic Stone Protein Predicts Outcome in Patients With Peritonitis in the ICU. Critical Care Medicine 41, 1027–1036 (2013).
- Garcıa de Guadiana-Romualdo, L., Albaladejo-Oton, M. D., Berger, M. & Trujillo-Santos, J. Prognostic performance of pancreatic stone protein in critically ill patients with sepsis. Biomarkers in Medicine.
- Vincent, J. L., Donadello, K. & Schmit, X. Biomarkers in the critically ill patient: C-reactive protein. Critical Care Clinical 27, 241–251 (2011).
- Van Singer, M. et al. Pancreatic stone protein for early mortality prediction in COVID-19 patients. Critical Care 25, 267 (2021)).
- Que, Y.-A. et al. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Critical care 16, R114 (2012).
- Vincent, J. L. et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med 26, 1793–1800 (1998).
- Boeck, L. et al. Pancreatic stone protein: a marker of organ failure and outcome in ventilator-associated pneumonia. Chest 140, 925–932 (2011).
- Schlapbach, L. J. et al. Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Medicine 39, 754–763 (2013).
- Jiří, Ž., Kýr, M., Vavřina, M. & Fedora, M. Pancreatic stone protein – A possible biomarker of multiorgan failure and mortality in children sepsis. Cytokine 66, 106–111 (2014).
- Rass, A. A. et al. The Role of Pancreatic Stone Protein in Diagnosis of Early Onset Neonatal Sepsis. BioMed Research International 2016, 1–8 (2016).
- Wu, Q., Nie, J., Wu, F., Zou, X. & Chen, F. Prognostic Value of High-Sensitivity C-Reactive Protein, Procalcitonin and Pancreatic Stone Protein in Pediatric Sepsis. Medical Science Monitor 23, 1533–1539 (2017).
- Peng, H.-Y., Zhu, Y.-M., Zhang, X.-P. & Kang, X.-Y. [Value of pancreatic stone protein/regenerating protein in severity evaluation and prognosis prediction for children with sepsis]. Zhongguo Dang Dai Er Ke Za Zhi 17, 1183–1188 (2015).*High interest publication ** Very high interest publication
Source: Abionic
Latest Articles
Pancreatic Stone Protein,PSP, Sepsis Test, Abioscope® Point-Of-Care Platform,Abionic
Sepsis is a life-threatening form of organ dysfunction caused by a dysregulated host response to infection1. This extremely heterogeneous and dynamic syndr...