Researchers discuss the significant impact of lung cancer globally, highlighting its high mortality rate and the importance of early detection for improved survival rates. Their paper mentions the effectiveness of low-dose CT (LDCT) screening in reducing lung cancer mortality, citing results from trials such as NLST and NELSON. However, it acknowledges the need for better methods to predict the behavior of lung nodules found during LDCT screening to minimize unnecessary follow-up tests and biopsies for benign nodules while detecting malignancies earlier.
The authors published a study in Radiology: Cardiothoracic Imaging to develop a radiomics-based reinforcement learning (S-RRL) model using serial LDCT scans. They aim to utilise this model to predict cancer progression effectively without waiting for follow-up interval testing, thereby enabling earlier detection of cancer and reducing unnecessary diagnostic procedures for benign nodules.
Developing a Radiomics-Based Reinforcement Learning Model for Early Cancer Detection
This retrospective study, conducted in compliance with the Health Insurance Portability and Accountability Act, involved 2500 anonymized individuals from the National Lung Screening Trial (NLST) project, spanning from August 2002 to April 2004. LDCT scans were collected from participants who underwent annual screening for up to 3 years. The study included 639 participants diagnosed with lung cancer and 1861 randomly selected participants without lung cancer. Among them, 1951 participants with noncalcified nodules measuring 4–30 mm in diameter at baseline were included. LDCT scans were acquired using scanners from four vendors, with different reconstruction settings. The participants were split into training/validation and test sets for analysis. The NLST protocol involved three screenings at 1-year intervals, with up to 7 years of additional follow-up. Participants diagnosed with lung cancer before the second screening were not offered subsequent tests, while those with indeterminate diagnoses were followed up with LDCT until the second screening.
Retrospective Study Design and Participant Characteristics
A total of 1951 participants were included in this study. In the training set (1404 participants; average age, 63 years [range, 55–74 years]; 818 male and 586 female participants), the average size of the nodules at baseline screening LDCT was 8.2 mm (range, 4–30 mm) in longest diameter. In the test set (547 participants; average age, 62 years [range, 55–74 years]; 311 male and 236 female participants), the average size of the baseline screening LDCT nodules was 9.6 mm (range, 4–30 mm) in longest diameter.
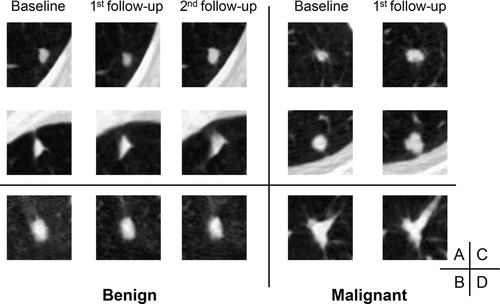
Image Source: Radiology: Cardiothoracic Imaging
Utilising Serial LDCT Scans to Capture Disease Progression Trajectory
The S-RRL model was designed to capture the trajectory of disease progression over time by analyzing serial LDCT scans. Unlike conventional models, which rely on comparisons with prior or follow-up scans, S-RRL utilized only serial examinations during training. This approach allowed the model to predict nodule progression without requiring additional scans, thus enabling early diagnosis at the baseline screening. By training the model with sequential LDCT scans acquired at multiple time points, the researchers leveraged the reinforcement learning method to discover the evolution of disease trajectory. This enabled more reliable assessment of malignancy risk for individual nodules, providing predictions not only at an early time point but also at future time points.
Outperforming Standard Models: The Superiority of the S-RRL Model
The study demonstrated that the S-RRL model outperformed standard models such as Lung-RADS and the Brock model in terms of overall performance for early lung cancer diagnosis at the time of baseline screening. Statistical analysis and clinical relevance showed that S-RRL significantly improved risk stratification, potentially impacting mortality through early intervention. However, the study had limitations, including its retrospective nature, subjective selection of malignant nodules, and modest improvements in predictive accuracy.
Future research is needed to enhance the model's performance and validate its clinical utility with larger datasets. Despite these limitations, the study suggests that exploiting the association between nodule progression and radiomics characteristics holds promise for improving lung cancer diagnosis.
Source: Radiology: Cardiothoracic Imaging
Image Credit: iStock