ICU Management & Practice, Volume 24 - Issue 3, 2024
Dyspnoea is among the worst suffering that a human being can experience. Because mechanically ventilated patients are strongly exposed to high dyspnoea intensity, it is important that clinicians monitor dyspnoea in this population. Relieving dyspnoea in patients is a human right.
Suffocating, not getting enough air or the feeling that breathing is difficult or even abnormal is among the worst suffering that a human being can experience. Because mechanically ventilated patients are at high risk of experiencing dyspnoea, the European Respiratory Society (ERS) and the European Society of Intensive Care Medicine (ESICM) decided a statement paper was required.
Recently, a multidisciplinary task force, with members from the ERS and the ESICM, including specialists in intensive care, respiratory intensive care, pulmonology, respiratory physiology, psychiatry, neurophysiology, and palliative care, together with a patient representative of the European Lung Foundation, addressed key issues related to the clinical problem of dyspnoea in critically ill mechanically ventilated patients. In addition to a systematic database search of medical literature, a patient-centred literature review was performed to explore the experiences of patients who had suffered dyspnoea while being mechanically ventilated for an acute illness. The manuscript was published in the European Respiratory Journal and Intensive Care Medicine (Demoule et al. 2024a; Demoule et al. 2024b).
Task Force Members first agreed on a new definition of dyspnoea, which is the symptom that conveys "an upsetting or distressing experience of breathing awareness". Previously, dyspnoea was defined by the American Thoracic Society as "a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity" (Parshall et al. 2012). Although clear, this definition was sophisticated and not operational enough at the bedside, and the word "discomfort" was probably too weak to describe the intensity of the distress/suffering associated with dyspnoea in invasively mechanically ventilated patients.
Clearly, reviewing the literature shows that dyspnoea is a frequent issue in invasively mechanically ventilated patients. In this population, the occurrence or intensity of dyspnoea has been investigated in approximately 50 studies, retrospective or prospective. Although these studies are extremely heterogeneous in terms of the design, it can be estimated that the median prevalence of mechanically ventilated patients who experience is approximately 45%. When present, dyspnoea ranges from 40 mm to 60 mm on a scale from zero (no dyspnoea) to 100 (worst imaginable dyspnoea). Altogether, these data show that dyspnoea in critically ill patients is frequent and rated as severe by patients. A similar level of pain would certainly be judged unacceptable by caregivers and would trigger an immediate response.
Why it is Essential to Monitor Dyspnoea at Bedside
In mechanically ventilated patients, dyspnoea has many consequences, which may occur either during the intensive care unit (ICU) stay or be delayed (Figure 1).
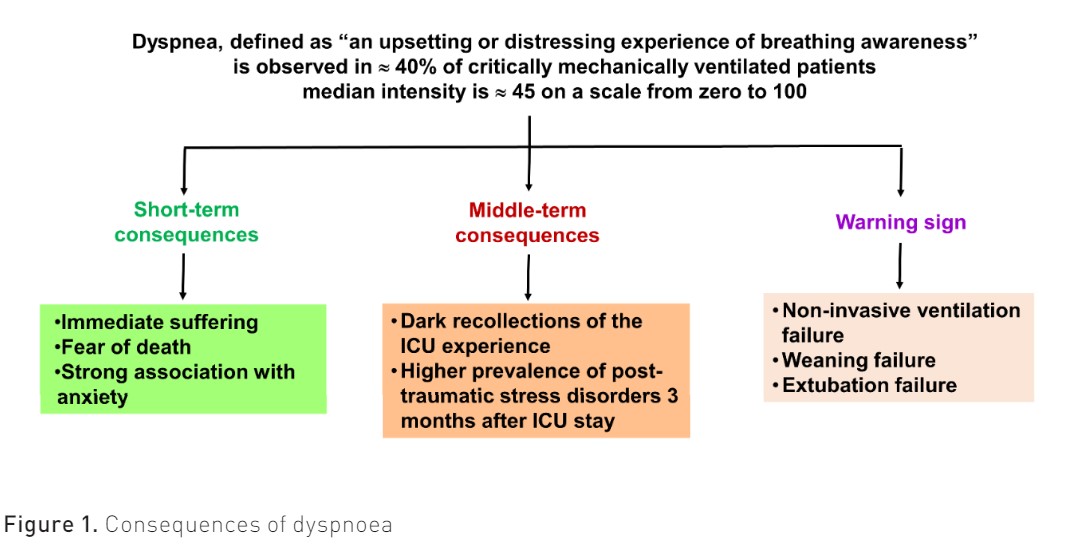
The first reason we should monitor dyspnoea is that it causes immediate respiratory suffering. Although it shares many similarities with pain, dyspnoea can be far worse than pain in that it is consistently associated with the fear of dying. The patient-centred literature review that the Task Force performed showed not only that dyspnoea is a terrifying sensation (“It’s hell. Not getting air” (Karlsson et al. 2012)) but also that dyspnoea is clearly associated with the fear of dying (“I felt like I was dying and didn’t get any air”) (Samuelson 2011), (“I often thought about death while I was attacked by dyspnoea”) (Shih and Chu 1999)). In addition, mechanically ventilated patients with dyspnoea are more likely to present with anxiety than non-dyspnoeic patients (71% vs. 24%) (Schmidt et al. 2011). Dyspnoea and anxiety are linked, so dyspnoea can generate or amplify anxiety, which in turn may amplify dyspnoea. Monitoring dyspnoea and reducing its intensity may, therefore, help reduce the terrible experience associated with this symptom.
Second, in addition to these short-term outcomes, dyspnoea contributes to the severe neuropsychiatric sequelae of ICU. Survivors of an ICU stay often carry extremely dark respiratory recollections of the experience of being mechanically ventilated, which may persist for several years. Among mechanically ventilated COPD patients, the worst recollection of ICU stay is poor sleep, after which comes suffocation, which is observed in 55% of patients (de Miranda et al. 2011). The combination of a distressing threat to life and a feeling of helplessness may generate post-traumatic stress disorder, which is observed in approximately 20% of ICU patients (Righy et al. 2019). In mechanically ventilated patients, the proportion of post-traumatic stress disorder 90 days after ICU admission is higher in those who experience dyspnoea (29%) than in those who did not (13%), and the repetition of dyspnoea episodes is strongly associated with post-traumatic stress disorder. Monitoring dyspnoea could help reduce the severe neuropsychiatric sequelae that dyspnoea contributes to generating.
The third reason we should monitor dyspnoea is because it is a warning sign. Indeed, dyspnoea is likely to result from a load capacity imbalance of the respiratory system. For instance, a higher level of dyspnoea seems to be associated with a higher risk of weaning failure (Decavèle et al. 2022a). During a spontaneous breathing trial, a high level of dyspnoea is associated with a higher level of failure (Bureau et al. 2022). In intubated patients, persistent dyspnoea, despite optimisation of ventilator settings, is associated with delayed extubation (Schmidt et al. 2011). Finally, once patients are extubated, a high level of dyspnoea, assessed two hours after extubation, is associated with a higher risk of post-extubation acute respiratory failure with subsequent need for re-intubation (Dres et al. 2021).
The Invisibility of Dyspnoea or Why Clinicians Often Ignore it in Patients
Dyspnoea is a symptom (as opposed to a physical sign) that places a very strong emphasis on self-reporting. The observation of signs of respiratory distress (e.g., tachypnoea and laboured breathing) may indicate the presence of dyspnoea, but these findings may be blunted by sedative or paralytic medications in mechanically ventilated patients. The inability to verbally or physically report a symptom does not mean it is not present, as clearly stated about pain (Raja et al. 2020). Data from many studies suggest that, for various reasons, the prevalence, intensity and impact of dyspnoea are underestimated by caregivers when assessing mechanically ventilated patients.
First, patients are not asked. There is a low level of awareness of this symptom within the ICU community. Actually, as opposed to pain, which each caregiver has experienced, very few caregivers have experienced dyspnoea (e.g. those with a chronic respiratory disease or who got near drowning) (Decavèle et al. 2022b). In addition, there are no guidelines that recommend routinely assessing dyspnoea in ICU patients. Finally, caregivers report that relieving dyspnoea presents a greater challenge than relieving pain (Gentzler et al. 2019).
Second, critically ill mechanically ventilated patients are frequently unable to self-report dyspnoea. The endotracheal limits vocal self-report of dyspnoea. In addition, other factors such as sedation, delirium or poor language may impair their ability to self-report dyspnoea. However, being noncommunicative does not mean that a patient is not suffering from dyspnoea. It only means that the patient cannot report it reliably. In other terms, the inability to communicate intentionally and reliably does not negate the possibility of experiencing dyspnoea.
Third, physicians, respiratory therapists and nurses fail to accurately assess dyspnoea based on their own observation of the patients they are managing (Binks et al. 2017; Gentzler et al. 2019; Haugdahl et al. 2015). For this reason, dyspnoea in mechanically ventilated patients may be characterised as “invisible”.In one study, where patients attributed a score of 50mm to dyspnoea on a visual analogue scale (VAS) from 0 to 100 mm, nurse and physician estimations were 20 mm (Haugdahl et al. 2015). The degree of underestimation increases as the patient's rating rises (Binks et al. 2017).
How to Detect Dyspnoea in Mechanically Ventilated Patients
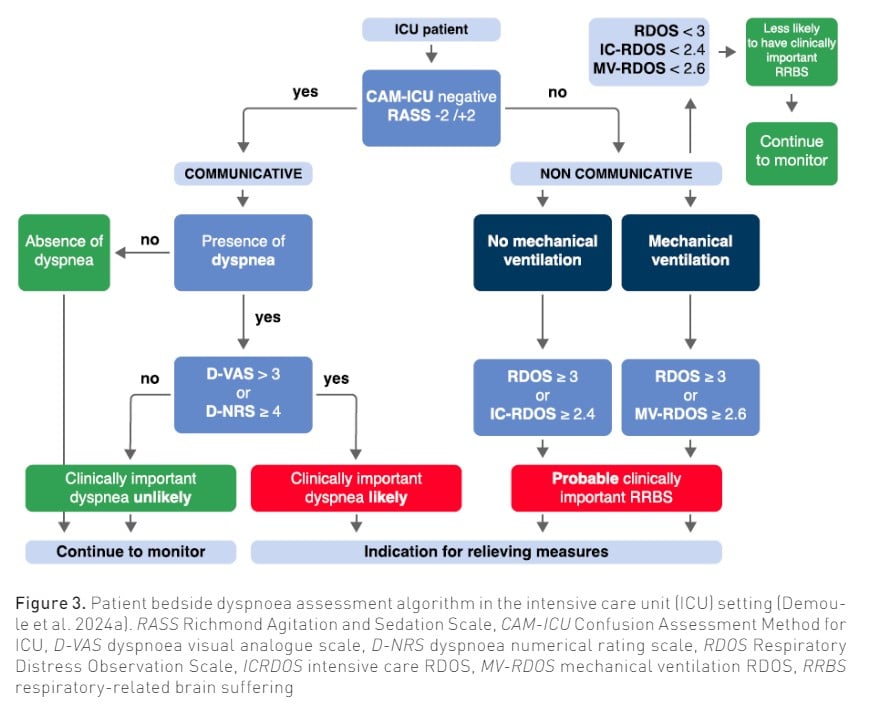
Like pain, the assessment of dyspnoea is based on self-report, which requires the patient to be communicative. In noncommunicative patients, observation scales or physiological markers can be used as dyspnoea surrogates.
Self-report of dyspnoea in communicative patients
As is the case with pain (Devlin et al. 2018), in order to self-report dyspnoea, the patient must be able to interpret sensory stimuli, pay attention to the clinician's instructions, concentrate to formulate a dyspnoea self-report and be able to communicate in some way. Unfortunately, less than 50% of patients receiving invasive mechanical ventilation are able to reliably self-report their symptoms (Demoule et al. 2022; Puntillo et al. 2010). Before searching for dyspnoea, it is therefore essential to assess whether a patient is able to reliably self-report a symptom.
The following approach is usually used to detect the presence of dyspnoea; the caregiver may employ dichotomous trigger questions, such as “Is your breathing comfortable?" Do you feel breathless? Is your breathing difficult? Are you getting enough air?” Consistency between the answers reinforces the conviction that self-report is reliable in a given patient. The last step is then to evaluate the intensity of dyspnoea. Although more than 40 tools are available to quantify the intensity of dyspnoea (Mularski et al. 2010), none are ideal for critically ill patients. The simplest way is probably to use a 0–10 numerical rating scale (NRS), which consists of determining, either verbally (asking between 0 and 10) (Morris et al. 2007) or visually (pointing a finger/mark on the 0–10 scale), which value corresponds to the patient’s dyspnoea intensity (Gift and Narsavage 1998). An alternative is the modified Borg category–ratio (0–10) scale that consists of verbal descriptors linked to specificnumbers (Burki 1987).
Inference of dyspnoea in noncommunicative patients
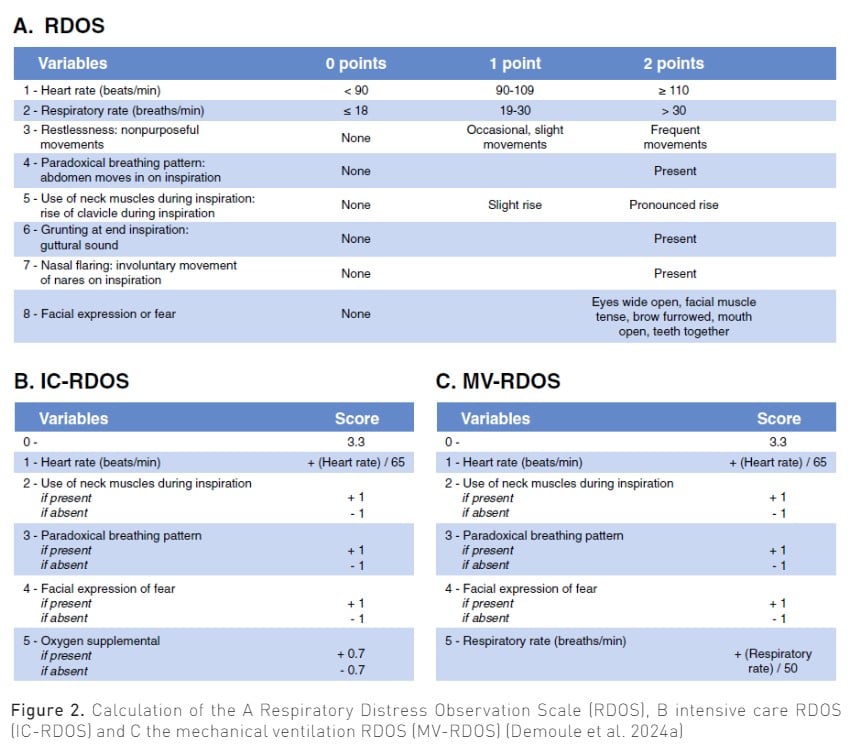
Observation scales have been initially developed to detect dyspnoea in noncommunicative patients (Campbell et al. 2017). They are based on observable signs of respiratory distress correlated with dyspnoea. The Intensive Care - Respiratory Distress Observation Scale (IC-RDOS) is a five-item ordinal scale, which infers the presence of dyspnoea based on three components: respiratory (use of neck muscles, paradoxical motion of the abdomen, need for oxygen), vegetative (heart rate) and emotional (facial expression of fear) (Decavèle et al. 2018a; Demoule et al. 2018; Persichini et al. 2015). It has a good inter-rater and scale reliability. More recently, the Mechanical Ventilation–Respiratory Distress Observation Scale (MV-RDOS) has been designed to be more adapted to intubated patients (Decavèle et al. 2023; Decavèle et al. 2018b). Figure 2 shows the main RDOS. An online calculator is available (https://dos-calc.pvsc.fr). Observation scales are an alternative way to identify dyspnoea when it cannot be self-reported.
Two electrophysiological indicators of dyspnoea are under evaluation in ICU patients. The first one is the electromyographic activity of the diaphragm (Decavèle et al. 2019) and extra-diaphragmatic inspiratory muscles (Schmidt et al. 2013). The second one is the electroencephalographic signatures of dyspnoea (Raux et al. 2019; Raux et al. 2007). These tools could help to detect and quantify dyspnoea regardless of the patient's level of cooperation. In the future, they could provide the opportunity for continuous monitoring of dyspnoea in intubated patients (Decavèle et al. 2023).
What is Clinically Important Dyspnoea?
Clinically important dyspnoea is defined as a dyspnoea-NRS ≥ 4, which corresponds to moderate intensity when compared to verbal descriptors (Wysham et al. 2015). By analogy with pain, a pain-NRS ≥ 4 is also the cut-off for moderate-to-severe pain and constitutes a clear indication for a prompt analgesic prescription (Devlin et al. 2018). Finally, dyspnoea-NRS ≥ 4 is associated with poorer outcomes (i.e. weaning failure, non-invasive ventilation failure and hospital mortality in patients receiving non-invasive ventilation (Bureau et al. 2022; Chen et al. 2017; Haugdahl et al. 2015; Dangers et al. 2018). However, in a study assessing whether a given level of dyspnoea is acceptable to patients, 30% of patients with ratings less than 4 considered their discomfort to be unacceptable (Stevens et al. 2019).
Regarding observational scales, an IC-RDOS score of 2.4 predicts a dyspnoea-VAS ≥ 4 with 72% sensitivity and 72% specificity (Persichini et al. 2015), and an MV-RDOS score of 2.6 in intubated patients predicted a dyspnoea-VAS>3 with 57% sensitivity and a 94% specificity (Decavèle et al. 2018b; Campbell et al. 2017).
Conclusion
Dyspnoea, an extremely distressing experience, is observed in approximately half of mechanically ventilated patients. When present, the intensity of dyspnoea is high. Dyspnoea has multiple deleterious consequences, including immediate suffering with a fear of dying and a strong association with anxiety. Dyspnoea also has long-term consequences, such as dark recollections of the ICU stay and a high prevalence of post-traumatic stress disorders. Like pain, dyspnoea is a self-reported symptom that imperfectly relates to physiological abnormalities. In mechanically ventilated patients able to communicate, the self-report of dyspnoea should be elicited as soon as possible during the ICU stay. In patients who are unable to communicate intentionally, it is possible to use an observational scale. When dyspnoea is present, the following interventions might be initiated to relieve it: reassurance of patients regarding their dyspnoea, reduction of non-respiratory stimuli of respiratory drive, minimisation of respiratory impedance and alterations of gas exchange, optimisation of ventilator settings, non-pharmacologic interventions such as air flux to face and relaxing music and, finally, a pharmacologic approach.
Conflict of Interest
Alexandre Demoule reports grants from the French Ministry of Health, Assistance Publique – Hôpitaux de Paris, Lungpacer, Respinor, consulting fees from Respinor, Lungpace, Lowenstein, Tribunal administrative de Cergy, Liberate Medical, payment or honoraria for lectures, presentations from Fisher & Paykel, Baxter, Getinge, Astra, Agence Européenne Informatique, Mindray, support for attending meetings and/or travel from Lungpacer, outside the submitted work. Maxens Decavèle reports support for attending meetings and/or travel from Isis medical outside the submitted work.
References:
Binks AP, Desjardin S, Riker R (2017) ICU clinicians underestimate breathing discomfort in ventilated subjects. Respir Care. 62:150–155.
Bureau C, Dres M, Morawiec E et al. (2022) Dyspnea and the electromyographic activity of inspiratory muscles during weaning from mechanical ventilation. Ann Intensive Care. 12:50.
Burki NK (1987) Dyspnea. Lung. 165:269–277.
Campbell ML, Kero KK, Templin TN (2017) Mild, moderate, and severe intensity cut points for the Respiratory Distress Observation Scale. Heart Lung J Crit Care. 46:14–17.
Chen Y-J, Hwang S-L, Li C-R et al. (2017) Vagal withdrawal and psychological distress during ventilator weaning and the related outcomes. J Psychosom Res. 101:10–16.
Dangers L, Montlahuc C, Kouatchet A et al. (2018) Dyspnoea in patients receiving non-invasive ventilation for acute respiratory failure: prevalence, risk factors and prognostic impact: A prospective observational study. Eur Respir J. 52.
de Miranda S, Pochard F, Chaize M et al. (2011) Postintensive care unit psychological burden in patients with chronic obstructive pulmonary disease and informal caregivers: A multicenter study. Crit Care Med. 39:112–118.
Decavèle M, Bureau C, Campion S et al. (2023) Interventions relieving dyspnea in intubated patients show responsiveness of the Mechanical Ventilation-Respiratory Distress Observation Scale. Am J Respir Crit Care Med. 208:39–48.
Decavèle M, Campbell ML, Persichini R et al. (2018) Management of Dyspnea in the Noncommunicative Patients: Consider Hetero-evaluation Scales. Chest. 154:991–992.
Decavèle M, Gay F, Persichini R et al. (2018) The Mechanical Ventilation-Respiratory Distress Observation Scale as a surrogate of self-reported dyspnoea in intubated patients. Eur Respir J. 52:1800598.
Decavèle M, Rozenberg E, Niérat M-C et al. (2022) Respiratory distress observation scales to predict weaning outcome. Crit Care. 26:162.
Decavèle M, Serresse L, Gay F et al. (2022) “Involve me and I learn”: an experiential teaching approach to improve dyspnea awareness in medical residents. Med Educ Online. 27:2133588.
Decavèle M, Similowski T, Demoule A (2019) Detection and management of dyspnea in mechanically ventilated patients. Curr Opin Crit Care. 25:86–94.
Demoule A, Decavele M, Antonelli M et al. (2024) Dyspnoea in acutely ill mechanically ventilated adult patients: an ERS/ESICM statement. Intensive Care Med. 50:159–180.
Demoule A, Decavele M, Antonelli M et al. (2024) Dyspnoea in acutely ill mechanically ventilated adult patients: an ERS/ESICM statement. Eur Respir J. 63:2300347.
Demoule A, Hajage D, Messika J et al. (2022) Prevalence, intensity, and clinical impact of dyspnea in critically ill patients receiving invasive ventilation. Am J Respir Crit Care Med. 205:917–926.
Demoule A, Persichini R, Decavèle M et al. (2018) Observation scales to suspect dyspnea in non-communicative intensive care unit patients. Intensive Care Med. 44:118–120.
Devlin JW, Skrobik Y, Gélinas C et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 46:e825–e873.
Dres M, Similowski T, Goligher EC, et al. (2021) Dyspnoea and respiratory muscle ultrasound to predict extubation failure. Eur Respir J. 58:2100002.
Gentzler ER, Derry H, Ouyang DJ et al. (2019) Underdetection and undertreatment of dyspnea in critically ill patients. Am J Respir Crit Care Med. 199:1377–1384.
Gift AG, Narsavage G (1998) Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 7:200–204.
Haugdahl HS, Storli SL, Meland B et al. (2015) Underestimation of patient breathlessness by nurses and physicians during a spontaneous breathing trial. Am J Respir Crit Care Med. 192:1440–1448.
Karlsson V, Lindahl B, Bergbom I (2012) Patients’ statements and experiences concerning receiving mechanical ventilation: a prospective video-recorded study. Nurs Inq. 19:247–258.
Morris NR, Sabapathy S, Adams L et al. (2007) Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 157:360–365.
Mularski RA, Campbell ML, Asch SM et al. (2010) A review of quality of care evaluation for the palliation of dyspnea. Am J Respir Crit Care Med. 181:534–538.
Parshall MB, Schwartzstein RM, Adams L et al. (2012) An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 185:435–452.
Persichini R, Gay F, Schmidt M et al. (2015) Diagnostic accuracy of respiratory distress observation scales as surrogates of dyspnea self-report in intensive care unit patients. Anesthesiology. 123:830–837.
Puntillo KA, Arai S, Cohen NH et al. (2010) Symptoms experienced by intensive care unit patients at high risk of dying. Crit Care Med. 38:2155–2160.
Raja SN, Carr DB, Cohen M et al. (2020) The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 161:1976–1982.
Raux M, Navarro-Sune X, Wattiez N et al. (2019) Adjusting ventilator settings to relieve dyspnoea modifies brain activity in critically ill patients: an electroencephalogram pilot study. Sci Rep. 9:16572.
Raux M, Ray P, Prella M et al. (2007) Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving non-invasive mechanical ventilation. Anesthesiology. 107:746–755.
Righy C, Rosa RG, da Silva RTA et al. (2019) Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care. 23:213.
Samuelson KAM (2011) Unpleasant and pleasant memories of intensive care in adult mechanically ventilated patients--findings from 250 interviews. Intensive Crit Care Nurs. 27:76–84.
Schmidt M, Demoule A, Polito A et al. (2011) Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 39:2059–2065.
Schmidt M, Kindler F, Gottfried SB et al. (2013) Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med. 39:1368–1376.
Shih FJ, Chu SH (1999) Comparisons of American-Chinese and Taiwanese patients’ perceptions of dyspnea and helpful nursing actions during the intensive care unit transition from cardiac surgery. Heart Lung J Crit Care. 28:41–54.
Stevens JP, Sheridan AR, Bernstein HB et al. (2019) A Multidimensional Profile of Dyspnea in Hospitalized Patients. Chest. 156:507–517.
Wysham NG, Miriovsky BJ, Currow DC et al. (2015) Practical dyspnea assessment: Relationship between the 0-10 numerical rating scale and the four-level categorical verbal descriptor scale of dyspnea intensity. J Pain Symptom Manage. 50:480–487.