ICU Management & Practice, Volume 24 - Issue 3, 2024
Every hospital can contribute to adequate lung donation. Learning and understanding the management of potential donors will allow them to receive proper care and be referred to save a life.
Introduction
Organ transplants are, in many cases, the only therapeutic option for patients with terminal diseases in different organs (Westphal et al. 2016). There is a marked imbalance between the number of available organs and the number of potential recipients (McKeown et al. 2012). In the U.K., U.S. and Europe, the number of potential transplant recipients has risen to more than 133,000, while the number of organs donated from all sources is not increasing enough to keep up with this growth rate (Klein et al. 2010).
Lung transplantation is a treatment option for people with terminal lung diseases despite the maximum medical treatment available. The number of transplants is limited by the shortage of organs, which generates high mortality on the waiting list. In Mexico, the population of patients with lung involvement likely to need a lung transplant is high. Chronic obstructive pulmonary disease (COPD) has a prevalence of 7.8% in the adult population. In idiopathic pulmonary fibrosis (IPF), an estimated annual incidence of up to eight cases per 100 thousand habitants is described (Moisés Acuña-Kaldman 2016; Monserrat Martínez Luna 2020).
According to data from the World Health Organization (WHO), our current health situation in relation to the COVID-19 pandemic, Mexico stands out as the seventh country with the most confirmed cases and the fourth with the most deaths, surpassing countries with a considerably higher population rate such as USA, Brazil and India (WHO 2023).
The urgent need to maintain lung transplant activity despite the pandemic is very clear, given the responsibility in our country to respond to the more than 20,000 patients waiting for an organ transplant. The National Centre of Transplants implemented on September 25, 2020 a coordinated gradual reactivation plan based on the control of the SARS-CoV-2 (COVID-19) epidemic in each federal entity (José Salvador Aburto-Morales 2020).
As in other parts of the world, the lack of donors in Mexico is a big problem. This, combined with multiple other factors, such as the lack of centres with adequate training and the cost, have prevented this procedure from being consolidated (Santillan-Doherty et al. 1993). Despite this, the first successful lung transplant for COVID-19 in Latin America was performed in Mexico by the only active group to date in this country (Wong-Jaén 2020).
Between April 2017 and December 2023, this lung transplant group performed 36 transplant procedures, 75% of them being two-lung. Twenty-five patients were men, and in 21 of the thirty-six patients, the diagnosis was idiopathic pulmonary fibrosis. Survival at 12 months was 78%, and at 90 days, it was above 85%.
The greatest challenges for lung transplantation in Mexico are not different from those faced by programmes in neighbouring countries with similar socioeconomic characteristics. It is vital to increase the rate of effective lung donation, increase the number of lung transplant programmes and overcome the learning curve.
Just to contextualise, it is known that in the best of scenarios, 40% of multi-organ donors will be able to effectively donate the lung (Klein et al. 2010). In relation to the population (130 million inhabitants), in 2019 Mexico exhibited an average global rate of organ donation due to brain death per million inhabitants of 4.5 (0.3 - 14.2), which as a result of the Sars Cov2 pandemic decreased for 2022 at 3.4 (0.5 - 11.1), a clearly low rate (CENATRA 2023).
Identification of the Multi-Organ Donor
Considering the shortage of organs available for transplant, it seems essential to inform every doctor who is dedicated to the care of critically ill patients that they have the tools for early identification and adequate and timely management of the potential donor in the areas that care for patients in critical condition, and not only in intensive care units (ICU) (van Zanden et al. 2019).
Any emergency room or intensive care unit can potentially house the next donor candidate. The prompt identification and adequate care of the potential donor is a task that any doctor who cares for critical patients (emergency doctor, intensivist, etc) must be able to carry out (Ismail et al. 2023). The fact that the hospital in question does not have a transplant or procurement programme is not a limitation in identifying potential donors through the Glasgow <7 programme (Bustos et al. 2006).
Glasgow <7 Programme
One-third of patients with Glasgow <7 progress to the criteria to be organ donors (Vanholder et al. 2021). Another third progresses to cardiopulmonary arrest, which makes them candidates to be tissue donors; the rest evolve towards improvement (Schoene et al., 2023). In any scenario, it is essential to provide adequate multiorgan support, in addition to establishing a neurological evaluation that includes performing a neurological window (Neitzke et al. 2019; Aulisio et al. 2007; Mizraji et al. 2009). Contrary to what one might think, starting these evaluations does not lead to a scenario of suspending support or preventing recovery scenarios (Aulisio et al. 2007). All three scenarios imply adequate care of the patient. What we are trying to avoid is the scenario where the support is suspended without considering the possibility that the patient may be a candidate for donation. If the patient is potentially a candidate for donation, a preliminary apnoea test without disconnection from the ventilator can be performed (Del Rio et al. 2009). If this test raises the possibility of the patient being a donor, it is important to contact the procurement centre (if the patient is not in one already) and discuss the possibility of transfer with the intention of increasing the patient's level of support and defining if the evolution will be towards improvement, or if the evaluation towards a potential donor can continue (Imaoka et al. 2023; Vail et al. 2023). While it is established if a patient in Glasgow <7 protocol evolves to any of the three previous scenarios, it is essential to maintain adequate multi-organ support (Messer et al. 2023).
General Non-Pulmonary Management of the Multi-Organ Donor
Haemodynamic
In a possible donor candidate, the main cause of hypotension is directly related to the cause of admission, and in most cases is hypovolaemia. This is where a balance must be found between aggressive resuscitation and fluid overload (ELAyashi et al. 2019; Marklin et al. 2023).
Hypotension is multifactorial (Chudoba et al. 2017). It is caused primarily by vasodilation associated with loss of vasomotor tone or spinal cord shock. Patients may also present hypovolaemia due to severe polyuria caused by diabetes insipidus or hypothermia. Finally, there may be hypotension of cardiogenic origin due to bradyarrhythmias or related to sepsis. Therefore, haemodynamic monitoring is mandatory for the appropriate differential diagnosis (Shah 2008; Darby et al. 1989).
Minimal monitoring (Rudnick et al. 2015; Kim et al. 2022b) requires measurement of the central venous pressure (CVP) and arterial line. It is desirable to maintain a target CVP >5 and <8 mmHg. In patients with great instability or high fluid need, monitoring of pulmonary artery pressure and cardiac output by thermodilution is recommended, especially for diagnosis and management of hypotension with vasopressors, volume and/or inotropes (Vieira and Carmona 2020). Pulmonary extravascular water can be monitored with a PiCCO catheter, maintaining <10 ml/kg ideal weight (Li et al. 2021). A non-invasive option is the use of lung ultrasound with the measurement of B lines maintaining an ultrasound extravascular water scale of less than 4 points (Lebovitz et al. 2016; Lindow et al. 2023). In the scenario of a possible donor candidate, the placement of a central venous catheter should never be an emergency; the initial resuscitation can be carried out via a peripheral catheter with enough time to plan the placement of a central access guided by ultrasound by an experienced provider (van der Mee-Marquet et al. 2023; Ouerd et al. 2023). In the case of diabetes insipidus or hypotension due to hypovolaemia caused by intense polyuria, resuscitation should be performed with hypotonic solutions or glucose solutions to reduce hypernatraemia (Opdam 2019; Meyfroidt et al. 2019; Kazemeyni and Esfahani 2008). In any other case, the use of balanced solutions is appropriate (Semler and Kellum 2019).
Endocrine
Once the criteria for brain death have been established, the initiation of steroids (15 mg/kg of methylprednisolone) should be considered due to the resulting pituitary adrenal insufficiency (Kuhn and Hahnenkamp 2019). There is no consensus on whether all cases require thyroid hormone replacement or the exact time for its initiation (Novitzky et al. 2014). Diabetes insipidus is prevalent during brain death and should be treated primarily with nasal desmopressin or vasopressin infusion based on urine output and serum sodium (Valenza et al. 2014).
Most consensuses propose serum glucose levels of the potential donor between 150 to 200 mg/dl. Glycaemic control will be complicated in the potential donor, especially if high doses of steroids are administered (Lagiewska et al. 1996). Only 1 in 4 donors will have glucose <200 mg/dl, and levels >250 mg/dl have been associated with failure in the donation process at some point. Another factor to keep in mind is that insulin doses can be >30 units/hour, especially in cases where steroid/thyroid hormone therapy is started or when hypotonic glucose solutions are used (Marvin and Morton 2009).
Haematologic
The ideal haemoglobin level for optimising oxygen transport to the organs to be transplanted is 10 g/dl (Kim et al. 2022a). Coagulation disorders and thrombocytopenia are common and worsen if there is hypothermia. They must be corrected according to the abnormality detected (Powner et al. 2011).
Thermal control
Normothermia is important for organ preservation. Furthermore, hypothermia causes vasodilation with hypotension, arrhythmias and coagulation disorders. Therefore, body temperature should always be maintained above 35 degrees Celsius with appropriate physical means (Wright et al. 2019).
Lung Donor
Lung donation represents greater difficulty than obtaining other solid organs due to several factors (Okahara et al. 2022):
- Lungs are the largest solid organs that are transplanted and are in direct contact with the atmosphere and, at the same time, with all the blood in the body.
- The condition of brain death is frequently due to multiple trauma, in which the possibility of chest trauma or bronchoaspiration is highly prevalent.
- The multi-organ donor in brain death is necessarily on mechanical ventilation with an artificial airway in an intensive care unit. The possibility of ventilator-associated pneumonia may make the lungs unsuitable for transplant.
Traditional lung donation candidate selection criteria are listed below (Chaney et al. 2014):
- Age < 55 years
- ABO blood type compatibility (RH compatibility is not necessary)
- Chest x-ray without opacities (rule out atelectasis)
- PaO2/FiO2 ≥300 with FiO2 100% PEEP 5 cmH2O after 20 min
- Smoking history < 20 pack years
- No history of chest trauma
- Minimal risk of aspiration or sepsis
- No history of cardiothoracic surgery
- Gram-negative bronchial secretion
- Absence of purulent secretions on bronchoscopy
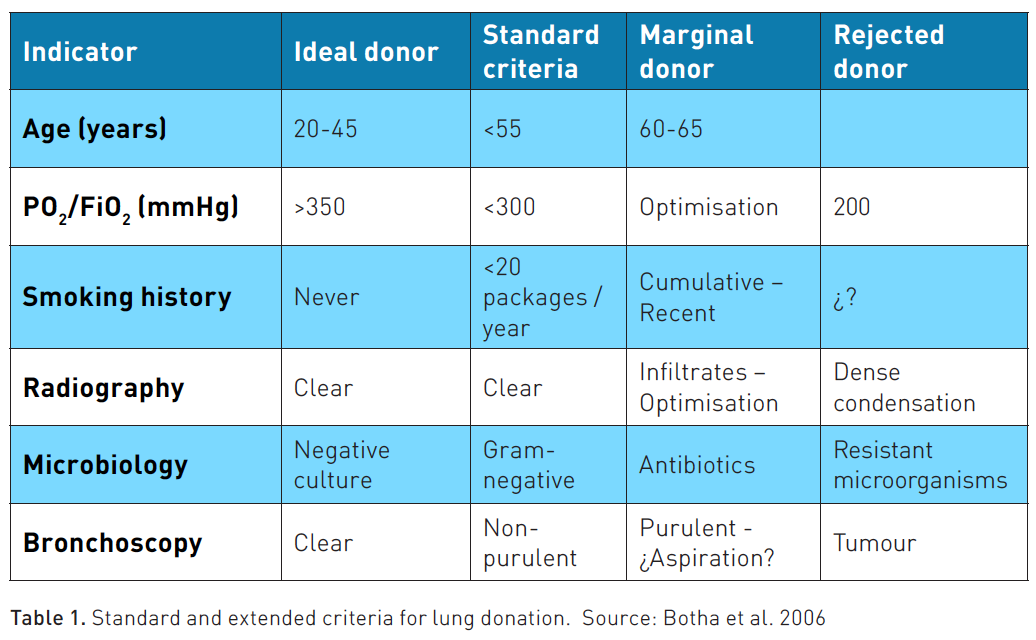
More than half of current donors worldwide do not meet half of these criteria. Thus, extended donor criteria have been proposed, and the success of these cases appears to be similar to that of candidates who meet the traditional criteria. In these cases, communication with a transplant expert is necessary to define whether the case is a candidate to be an extended donor and not rule out any potential donor (Minambres et al. 2016). Table 1 shows the extended criteria that have shown a very similar long-term evolution in patients transplanted from marginal donors. Many of them can be rescued based on protocols that optimise the organs, which are shown below (Lesko and Angel 2023; Botha et al. 2006).
On the other hand, the anthropometric characteristics between donor and recipient should be compared (Ogunlana et al. 2021), with the most used formula being the prediction of total lung capacity (TLC) see formulas 1 and 2 (Barnard et al. 2013):
Women:
TLC = (7.99 * m) – 7.08
Men:
TLC = (6.6 * m) – 5.79
where: m = stature in metres
The donor and recipient must share between 75-125% of the CPT. This range is very wide due to the recipient's ability to adapt the thoracic cavity to the new lung. However, in complex cases when the recipient's thoracic cavity is too small, graft reduction adjustments should be evaluated. The primary recruitment centres for potential donors will not carry out the comparison, but this is very important information that the transplant group must have in the initial evaluation data of the case (Ouwens et al. 2002). Another widely used method is to use chest measurements, which are possible with digital imaging equipment, between the recipient's x-ray and the donor's x-ray.
In lung transplantation, ABO blood group compatibility is necessary. There is no difference in the results when compatible and non-identical donors are used, such as in blood transfusion. Rh compatibility is not considered in lung transplantation (Chen-Yoshikawa 2023).
Regarding ischaemia time, the time from the donor's aortic clamping to begin procurement until the restart of reperfusion in the transplanted lung should be around six hours, an important logistical aspect to consider the procurement, transfer and implantation time. Only a third of cases worldwide report these times (Meyer et al. 2000).
Care of the Lung Donation Candidate
If an adequate care protocol is not established, especially regarding mechanical ventilation, donation candidates may only be accepted in <25% of cases, while if appropriate care is applied, 1 in 2 candidates may be procured (Shepherd et al. 2021).
Fluid management is crucial in pulmonary procurement. Unlike other organs such as the kidney, lungs suffer damage such as inflammation and oedema if there is any excess in body volume. The haemodynamic monitoring methods to achieve this objective have already been mentioned in previous sections.
Patients should be managed ventilatorily with lung protection criteria. The tidal volume should be 6-8 ml/kg of ideal body weight with the minimum FiO2 that maintains appropriate oxygen saturation. The PEEP level can be set between 5 and 8 cmH2O. Patients can be managed in pressure or volume mode with an appropriate respiratory rate to maintain normal pH and pCO2.
It is extremely important that during aspiration of secretions there is no desaturation using a closed aspiration system. Aspirate only if there are secretions and avoid inserting the aspiration catheter beyond the carina.
It will always be desirable to perform a bronchoscopy on the donor. This procedure helps cleaning the airway and bronchi. In addition, it helps to evaluate if there is evident infection of the airway and lung parenchyma. It is normal to find purulent secretions in the bronchi that may mean bronchitis. However, if bronchoscopy detects distal secretions that continue to be aspirated continuously, this suggests that there may be distal infection or pneumonia, which rules out that lung for donation (Figure 1).
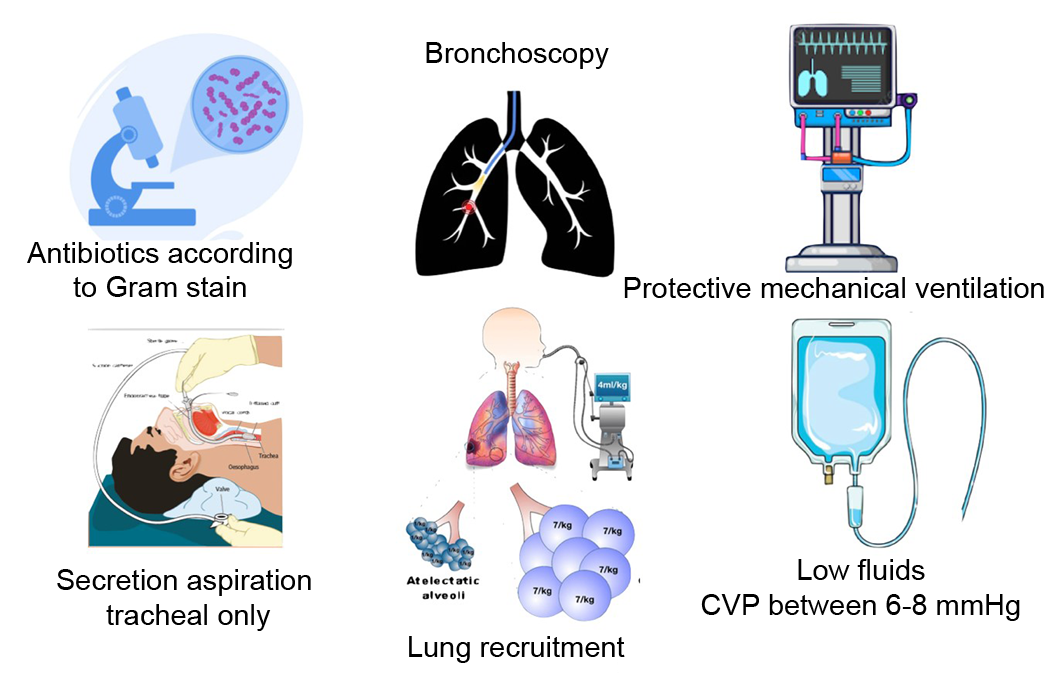
Figure 1. Lung donor care measures
Different rescue protocols for marginal lungs have been published. The reasoning behind these protocols is to maximise lung function through fluid management and bronchial cleansing by bronchoscopy, in addition to the use of alveolar recruitment manoeuvres with mechanical ventilation. Thus, there are lungs that may not have acceptance criteria for lung transplantation but can be optimised or rescued with these protocols. The first publication in this regard is the “SALT” protocol from the group at the University of Texas at San Antonio (Angel et al. 2006). The suggested recruitment is to place the patient in a pressure mode on the ventilator, with PEEP of 15 cmH2O and cycling pressure of 15 cmH2O. Some lungs can improve their pO2/FiO2 ratio with this management in addition to bronchoscopy and fluid optimisation.
Very recently, ex-vivo perfusion has been proposed using perfusion and extracorporeal ventilation for a few hours to rescue these lungs, even using antibiotic therapy in case of infection (Sommer et al. 2013; Snell et al. 2018).
Conclusion
Figure 2 shows an infographic with a summary of all the points discussed here. It is based on the SOSD mnemonic, which is useful to consider all aspects of multi-organ donation with a focus on the lung donor. There are very few hospitals that have the capacity to have a lung transplant programme. Hospitals with the capacity to procure organs are not sufficient for the number of donors required. Therefore it is important that all hospitals can accommodate a potential donor, and it is important that all potential donor candidates can have an adequate evaluation and, if applicable, they can be integrated into a donation programme. If your hospital does not have a procurement programme, Glasgow <7 patients should be identified and proposed to be sent to a centre with experience and authorisation for organ procurement.
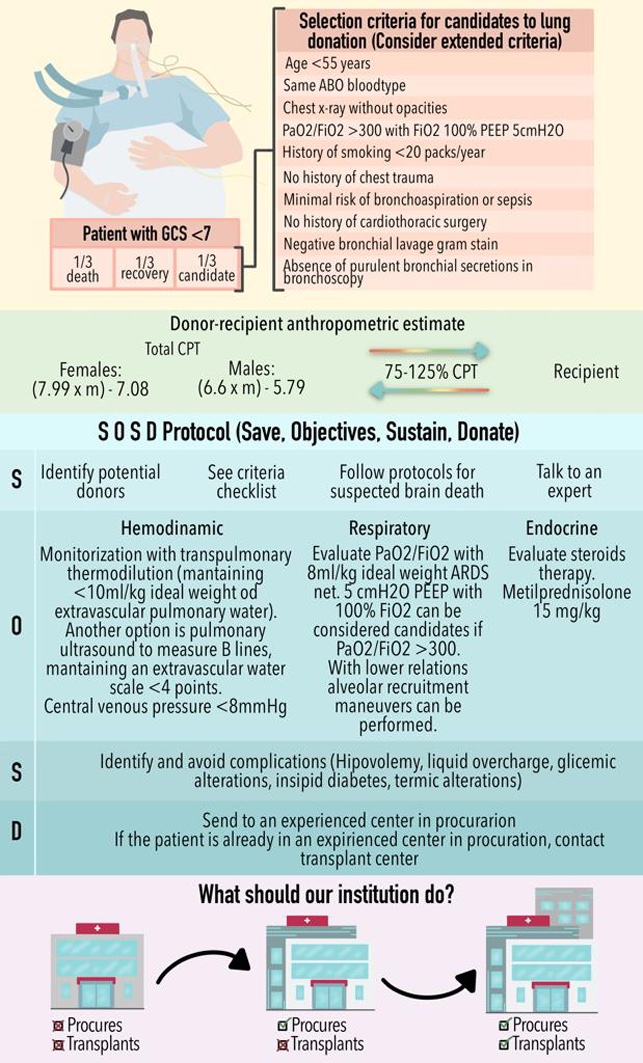
If it is a procurement centre, the evaluation of the potential candidate must be completed, and a lung transplant programme and a centre with experience in organ transplantation must be contacted. Time is vital since brain death has a time window limited to a few days. Organ donation should be part of any hospital. This way, the organ procurement rate in our country can be improved, thus benefitting thousands of patients who would have the opportunity to receive the gift of life.
Conflict of Interest
None.
References:
Angel LF et al. (2006) Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 174(6):710-716.
Aulisio MP, Devita M, Luebke D (2007) Taking values seriously: Ethical challenges in organ donation and transplantation for critical care professionals. Crit Care Med. 35(2 Suppl):S95-101.
Barnard JB et al. (2013) Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. 32(9):849-860.
Botha P et al. (2006) Extended donor criteria in lung transplantation: impact on organ allocation. J Thorac Cardiovasc Surg. 131(5):1154-1160.
Bustos JL, Surt K, Soratti C (2006) Glasgow coma scale 7 or less surveillance program for brain death identification in Argentina: Epidemiology and outcome. Transplant Proc. 38(10):3697-3699.
CENATRA. Boletín Estadístico Informativo Centro Nacional de Trasplantes [online]. Available at https://www.gob.mx/cenatra/documentos/boletin-estadistico-informativo?idiom=es
Chaney J et al. (2014) Lung donor selection criteria. J Thorac Dis. 6(8):1032-1038.
Chen-Yoshikawa TF (2023) ABO blood type incompatible lung transplantation. J Thorac Dis. 15(6):3437-3442.
Chudoba P et al. (2017) Brain death-associated pathological events and therapeutic options. Adv Clin Exp Med. 26(9):1457-1464.
Darby JM et al. (1989) Approach to management of the heartbeating 'brain dead' organ donor. JAMA. 261(15):2222-2228.
Del Rio F et al. (2009) Evaluation and maintenance of the lung donor. Med Intensiva. 33(1):40-49.
ELAyashy M E et al. (2019) The validity of central venous to arterial carbon dioxide difference to predict adequate fluid management during living donor liver transplantation. A prospective observational study. BMC Anesthesiol. 19(1):111.
Imaoka Y et al. (2023) Breaking distance barriers in liver transplantation: Risk factors and outcomes of long-distance liver grafts. Surgery.
Ismail AJB et al. (2023) Barriers to the identification of possible organ donors among brain-injured patients admitted to intensive care units. Korean J Transplant. 37(2):85-94.
José Salvador Aburto-Morales JR-M, Cinthya Ayerim Lucio-García, José André Madrigal-Bustamante (2020) Mexico’s response to COVID-19 epidemic (SARS-CoV-2) and the recommendations to the National Donation and Transplant Subsystem. Revista Mexicana de Trasplantes. 9(1):6-14.
Kazemeyni SM, Esfahani F (2008) Influence of hypernatremia and polyuria of brain-dead donors before organ procurement on kidney allograft function. Urol J. 5(3):173-177.
Kim S et al. (2022) Evaluation of red blood cell transfusion threshold in the management of brain-dead organ donors. Medicine (Baltimore). 101(50):e32353.
Kim UR et al. (2022) Central Versus Peripheral Invasive Arterial Blood Pressure Monitoring in Liver Transplant Surgery. Cureus. 14(12):e33095.
Klein AS et al. (2010) Organ donation and utilization in the United States, 1999-2008. Am J Transplant. 10(4 Pt 2):973-986.
Kuhn SO, Hahnenkamp K (2019) Critical care management of the potential organ donor : Current recommendation for adults. Med Klin Intensivmed Notfmed. 114(2):132-138.
Lagiewska B et al. (1996) Hemodynamic and metabolic disturbances observed in brain-dead organ donors. Transplant Proc. 28(1):165-166.
Lebovitz DJ et al. (2016) Lung Ultrasound Utility in the Management of the Neurologically Deceased Organ Donor. Prog Transplant. 26(3):210-214.
Lesko MB, Angel LF (2023) Organ Donation, the Non-Perfect Lung Donor, and Variability in Conversion to Transplant. Clin Chest Med. 44(1):69-75.
Li X et al. (2021) Extravascular Lung Water and Intrathoracic Blood Volume Index Are Associated With Liver Function in Brain Dead Donors for Organ Transplant. Exp Clin Transplant. 19(5):450-456.
Lindow T, Quadrelli S, Ugander M (2023) Noninvasive Imaging Methods for Quantification of Pulmonary Edema and Congestion: A Systematic Review. JACC Cardiovasc Imaging. 16(11):1469-1484.
Marklin GF
et al. (2023) Clinical outcomes of a prospective randomized comparison of
bioreactance monitoring versus pulse-contour analysis in a stroke-volume based
goal-directed fluid resuscitation protocol in brain-dead organ donors. Clin
Transplant. e15110.
Marvin MR, Morton V (2009) Glycemic control and organ transplantation. J Diabetes Sci Technol. 3(6):1365-1372.
McKeown DW, Bonser RS, Kellum JA (2012) Management of the heartbeating brain-dead organ donor. Br J Anaesth. 108 Suppl 1:i96-107.
Messer S et al. (2023) A national pilot of donation after circulatory death (DCD) heart transplantation within the United Kingdom. J Heart Lung Transplant. 42(8):1120-1130.
Meyer DM et al. (2000) Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest. 118(5):1255-1262.
Meyfroidt G et al. (2019) Management of the brain-dead donor in the ICU: general and specific therapy to improve transplantable organ quality. Intensive Care Med. 45(3):343-353.
Minambres E et al. (2016) An intensive lung donor treatment protocol does not have negative influence on other grafts: a multicentre study. Eur J Cardiothorac Surg. 49(6):1719-1724.
Mizraji R et al. (2009) Brain death epidemiology in Uruguay and utilization of the Glasgow coma score in acute brain injured patients as a predictor of brain death. Transplant Proc. 41(8):3489-3491.
Moisés Acuña-Kaldman YA-N, Alejandro Arreola-Morales, Alma Bizarrón-Muro et al. (2016) Primer Consenso Mexicano sobre Fibrosis Pulmonar Idiopática. NCT Neumología y Cirugía de Tórax. 75(1):32-51.
Monserrat Martínez Luna AR-G, Ricardo Isidro Lázaro Pacheco, José Enrique Meza Alvarado et al (2020) Chronic Obstructive Pulmonary Disease (COPD). Bases for the General Practitioner. Revista de la Facultad de Medicina (México). 63(3):28-35.
Neitzke G et al. (2019) Decision-making support in Intensive Care to facilitate organ donation : Position paper of the Ethics Section and the Organ Donation and Transplantation Section of the German Interdisciplinary Association of Critical Care and Emergency Medicine (DIVI) in collaboration with the Ethics Section of the German Society of Medical Intensive Care Medicine and Emergency Medicine (DGIIN). Med Klin Intensivmed Notfmed. 114(4):319-326.
Novitzky D et al. (2014) Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: a retrospective analysis. Transplantation. 98(10):1119-1127.
Ogunlana MO et al. (2021) Anthropometric determinants of lung function in apparently healthy individuals. S Afr J Physiother. 77(1):1509.
Okahara S et al. (2022) An Audit of Lung Donor Pool: Optimal Current Donation Strategies and the Potential of Novel Time-Extended Donation After Circulatory Death Donation. Heart Lung Circ. 31(2):285-291.
Opdam HI (2019) Hormonal Therapy in Organ Donors. Crit Care Clin. 35(2):389-405.
Organization WH. WHO Coronavirus (COVID-19) Dashboard [online]. Available at https://covid19.who.int/?mapFilter=cases
Ouerd S et al. (2023) Vasopressin Use in the Support of Organ Donors: Physiological Rationale and Review of the Literature. Crit Care Explor. 5(4):0907.
Ouwens JP et al. (2002) Size matching in lung transplantation using predicted total lung capacity. Eur Respir J. 20(6):1419-1422.
Powner DJ, Allison TA, Zakaria A (2011) Advanced assessment of platelet function during adult donor care. Prog Transplant. 21(3):228-235.
Rudnick MR, Marchi LD, Plotkin JS (2015) Hemodynamic monitoring during liver transplantation: A state of the art review. World J Hepatol. 7(10):1302-1311.
Santillan-Doherty P et al. (1993) Lung procurement in Mexico. Transplant Proc. 25(6):3137-3138.
Schoene D et al. (2023) Identification of patients at high risk for brain death using an automated digital screening tool: a prospective diagnostic accuracy study. J Neurol. 270(12):5935-5944.
Semler MW, Kellum JA (2019) Balanced Crystalloid Solutions. Am J Respir Crit Care Med. 199(8):952-960.
Shah VR (2008) Aggressive management of multiorgan donor. Transplant Proc. 40(4):1087-1090.
Shepherd HM et al. (2021) Advanced considerations in organ donors. J Thorac Dis. 13(11):6528-6535.
Snell GI et al. (2018) Donation after Brain Death versus Donation after Circulatory Death: Lung Donor Management Issues. Semin Respir Crit Care Med. 39(2):138-147.
Sommer W et al. (2013) Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J Heart Lung Transplant. 32(11):1065-1072.
Vail EA et al. (2023) Deceased Organ Donor Management and Organ Distribution From Organ Procurement Organization-Based Recovery Facilities Versus Acute-Care Hospitals. Prog Transplant. 33(4):283-292.
Valenza F et al. (2014) A standardized model of brain death, donor treatment, and lung transplantation for studies on organ preservation and reconditioning. Intensive Care Med Exp. 2(1):12.
van der Mee-Marquet N et al. (2023) Ultrasound guidance practices used for the placement of vascular accesses in intensive care units: an observational multicentre study. Eur J Med Res. 28(1):528.
van Zanden JE et al. (2019) Complement Therapeutics in the Multi-Organ Donor: Do or Don't? Front Immunol. 10:329.
Vanholder R et al. (2021) Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 17(8):554-568.
Vieira RF, Carmona MJC (2020) Volemia and kidney transplantation. Braz J Anesthesiol. 70(3):191-193.
Westphal GA et al. (2016) The effect of brain death protocol duration on potential donor losses due to cardiac arrest. Clin Transplant. 30(11):1411-1416.
Wong-Jaén M, Chavarría-Martínez U, Fuentes-Puga V et al. (2020) Trasplante pulmonar en México en tiempo de pandemia por COVID-19. Rev Mex Cir Torac Gen. 1(2):67-72. 9.
Wright C et al. (2019) The Impact of Therapeutic Hypothermia Used to Treat Anoxic Brain Injury After Cardiopulmonary Resuscitation on Organ Donation Outcomes. Ther Hypothermia Temp Manag. 9(4):258-264.