ICU Management & Practice, Volume 24 - Issue 3, 2024
Haemodynamic instability and shock are a potential everyday challenge for intensivists and anaesthesiologists. Understanding the underlying cause is pivotal for an appropriate and successful treatment. Systolic motion of the anterior mitral valve leaflet towards the left ventricular outflow tract (SAM) is a possible insidious mechanism of low cardiac output, severe haemodynamic instability, and hypoxaemia. All anaesthesiologists and intensivists should be aware of the mechanisms, risk factors, diagnostic features, and treatment of SAM.
Introduction
Haemodynamic instability and shock are a potential everyday challenge for intensivists and anaesthesiologists. Understanding the underlying cause is pivotal for an appropriate and successful treatment. Although cardiac output (CO) monitoring is often a first step in the management of haemodynamic instability/shock, allowing differential diagnosis between vasoplegia/distributive shock and low CO states, the causes and mechanisms of the latter can be multiple, and not all are easily recognisable if not actively researched. Systolic motion of the anterior mitral valve leaflet towards the left ventricular outflow tract (LVOT), usually more simply referred to as Systolic Anterior Motion (SAM), is a possible insidious mechanism of low CO, severe haemodynamic instability and hypoxaemia (due to dynamic LVOT obstruction and acute mitral regurgitation) requiring an echocardiographic diagnosis and a rather counterintuitive treatment (Guigui et al. 2022; Sherrid et al. 2016; Slama et al. 2016; Uematsu et al. 2017).
Although initially described as a feature of hypertrophic cardiomyopathy (HCM) (Guigui et al. 2022), SAM can also occur in the absence of pre-existing heart disease, especially in association with the haemodynamic changes which often occur in the perioperative and critical care setting, such as hypovolaemia, vasoplegia, and tachycardia (Dugar et al. 2016; Chauvet et al. 2015; Mingo et al. 2006; Abbas et al. 2019; Raut et al. 2018; Luckner et al. 2005). For this reason (and, of course, because patients with HCM may undergo surgery or be admitted to an intensive care unit for any other disease), all anaesthesiologists and intensivists should be aware of the mechanisms, risk factors, diagnostic features, and treatment of SAM.
What is Systolic Anterior Motion?
SAM is defined as the displacement of the anterior mitral leaflet (AML) towards the LVOT during systole, causing dynamic LVOT obstruction (LVOTO). It can result in mitral regurgitation (MR), reduction in CO, pulmonary oedema, hypotension, and shock (Guigui et al. 2022; Slama et al. 2016; Dugar et al. 2016; Raut et al. 2018; Cresci 2017; Duncan et al. 2023).
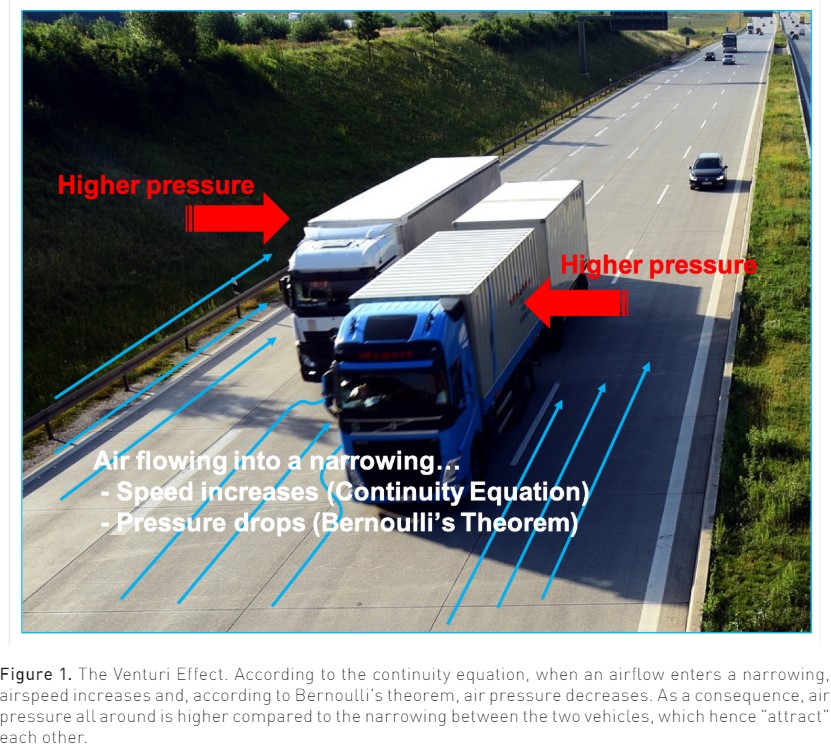
The mechanism of SAM is traditionally attributed to the so-called Venturi Effect, that is, the drop in pressure occurring when a fluid flows through a narrowed orifice (Pisano 2021). More precisely, what is called the Venturi Effect is the combined effect of two physical laws: the continuity equation (or Leonardo's law, after Leonardo Da Vinci) and Bernoulli's theorem (Pisano 2021). The continuity equation states that the product of the speed v of an ideal fluid flowing in a conduit and of the cross-sectional area A of the conduit itself, namely the volume flow rate Q, remains constant all along the conduit:
Q = A v = a constant
Accordingly, if the cross-sectional area decreases, the fluid speed must increase. An everyday example of this law is the increase in the speed of water coming out of a garden hose when you partially close the hose end with your thumb.
The Bernoulli's theorem is a little more complex. However, it can simply be said that when elevation changes are neglected, considering a fluid flowing along a horizontal streamline, the pressure is lower where the speed is higher, and vice versa. Airplanes can fly thanks to this principle (Pisano 2021).
The combined effect of these two physical laws causes that when a fluid flows through a narrowing, its speed increases (the continuity equation) and, accordingly, its pressure decreases (Bernoulli's theorem). Figure 1 shows an example of the Venturi Effect that anyone can experience on the highway: when one vehicle comes very close to another during a high-speed overtaking, the two vehicles attract each other. In particular, if a car is overtaking a truck, it is the (lighter) car that is attracted towards the truck. A similar thing can happen in the left ventricle in the presence of all those conditions that cause LVOT narrowing or, anyway, increase blood speed through it, leading to a pressure drop which exerts a suction force dragging the AML into the LVOT towards the interventricular septum during systole (Dugar et al. 2016; Luckner et al. 2005; Pisano 2021).
Alternatively, the drag effect hypothesises that, in predisposed patients, the mitral valve (MV) leaflets are positioned in the path of LVOT flow, which drags them anteriorly and superiorly toward the septum (Dugar et al. 2016). This diastolic anterior motion of the MV may start even before the onset of systole when the velocities in the LVOT are still low (Guigui et al. 2022; Levine et al. 2014; Ro et al. 2014).
Predisposing and Precipitating Factors
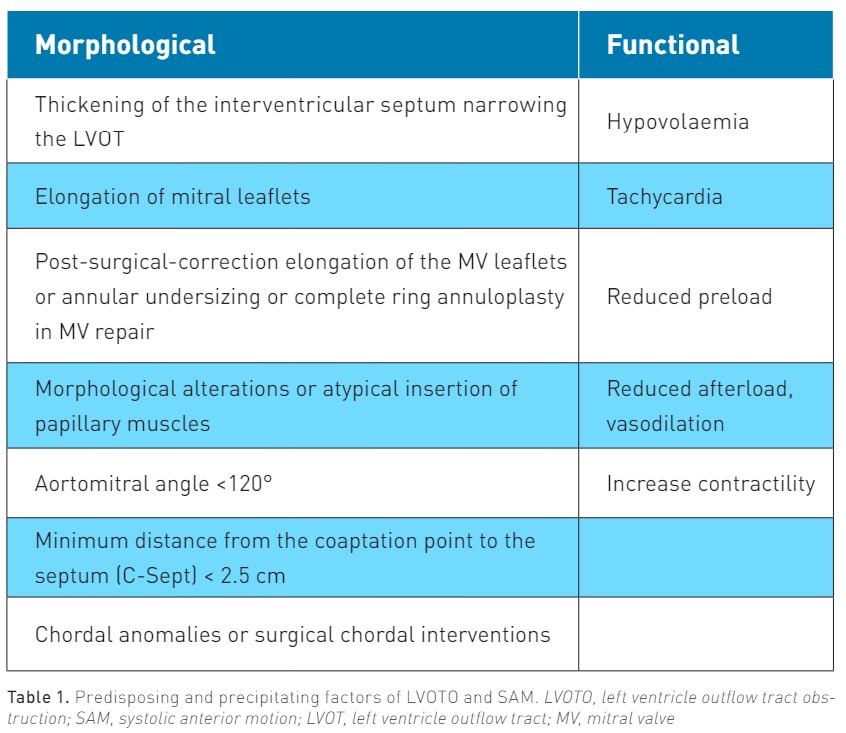
Table 1 shows the predisposing (morphological) factors of SAM and all conditions that may precipitate SAM in the presence or absence of such predisposing factors.
As mentioned, SAM and LVOTO are most often observed in HCM. HCM is a rare but potentially life-threatening disease affecting around 1:200 to 1:500 individuals in the general population (Guigui et al. 2022). It is characterised by left ventricular (LV) hypertrophy without pressure overload and is a possible cause of sudden death. About 60% to 70% of patients with HCM have either resting or provocable SAM of the MV and LVOTO (Guigui et al. 2022; Cresci 2017). In HCM, reduced LV cavity dimensions, myocardial hypertrophy, and increased LV contractility are all factors that increase blood speed in the LVOT during systole, predisposing to SAM. More generally, global or isolated (septal) hypertrophy represents a predisposing factor for SAM: while a diastolic ventricular septum thickness >15 mm is considered diagnostic for HCM (Uematsu et al. 2017; Cresci 2017), a sigmoid septum is regarded to as a risk factor for SAM also in patients without HCM (Uematsu et al. 2017).
However, SAM has been described in patients with or without myocardial hypertrophy in a variety of clinical settings, such as general or neuraxial anaesthesia (Chou 2022; Fujita et al. 2015; Hussey et al. 2023; Monaco et al. 2022; Essandoh et al. 2016), cardiac surgery (particularly after MV repair or aortic valve replacement) (Weich et al. 2021; Makhija et al. 2019; Ashikhmina et al. 2021), myocardial infarction (Mingo et al. 2006), septic shock (Chauvet et al. 2015; Mingo et al. 2006; Abbas et al. 2019; Balik et al. 2020), use of inotropic medications (Mingo et al. 2006), exercise or dobutamine stress test (Alhaj et al. 2013), and anaphylaxis (Nooli et al. 2023). Finally, dynamic LVOTO has also been described in the acute phase of Tako-Tsubo cardiomyopathy (Yasuhiro et al. 2022; Di Vece et al. 2021; Conradi et al. 2021). In all these clinical settings, one or more factors, including hypovolaemia, vasodilation, increased heart rate, and increased inotropism (Dugar et al. 2016), may contribute to increasing blood velocity through the LVOT and, hence, to SAM development.
SAM in the Intensive Care Unit and Perioperative Setting
Intensive Care Unit (ICU) patients, as well as patients undergoing surgery, are particularly exposed to haemodynamic changes that may increase blood velocity through the LVOT, possibly leading (or contributing) to SAM development, such as hypovolaemia (e.g. acute blood loss), vasodilation (e.g. distributive shock, neuraxial anaesthesia, use of vasodilator drugs), tachycardia (e.g. compensatory, pain, fever, anxiety), or increased LV contractility (e.g. use of inotropic drugs, hyperdynamic shock) (Slama et al. 2016). However, reports of haemodynamic instability/shock due to SAM in these settings are mostly limited to case reports or small case series.
SAM in ICU patients
Dynamic LVOTO is a potential cause of severe haemodynamic instability, despite volume optimisation and use of vasoconstrictors, in patients with septic shock (Dugar et al. 2016; Chauvet et al. 2015; Balik et al. 2020; Evans et al. 2021). Among 218 patients admitted to ICU due to septic shock, Chauvet et al. (2015) reported the presence of echocardiographic signs of left intraventricular dynamic obstruction in 22% and found that this occurrence was associated with higher 28-day mortality. However, Balik et al. (2020) found severe LVOTO (including SAM) in only 10 out of 527 septic shock patients. Other authors reported SAM as the main mechanism of severe shock in septic patients without pre-existing heart disease, possibly due to vasodilation/tachycardia/increased LV contractility associated with sepsis (e.g. Dugar et al. 2016). In other cases, the occurrence of SAM in two ICU patients was attributed to an excessive use of catecholamines (Mingo et al. 2006).
A poor response to treatment with inotropic agents and/or mechanical circulatory support after myocardial infarction should always prompt echocardiographic evaluation in order to rule out LVOTO, which can mimic cardiogenic shock: also in this case, hypercontractility of non-ischaemic regions, changes in LV chamber size, and tachyarrhythmias (e.g. following catecholamine administration) can lead to dynamic LVOTO (Mingo et al. 2006; Harrington et al. 2023).
Finally, dynamic LVOTO has been described in the setting of neurosurgical ICU in two patients treated with milrinone (an inodilator) for cerebral vasospasm, with confirmed SAM in at least one of the two cases (Baulier et al. 2022).
SAM in the perioperative setting
Dynamic LVOTO/SAM is a well-known phenomenon to cardiac anaesthesiologists. After cardiac surgery, it can especially occur following aortic valve replacement (AVR) or MV repair (MVR). In patients undergoing AVR, in addition to the factors mentioned above such as LV septal hypertrophy, use of inotropic medications, hypovolaemia, and vasodilation, further predisposing factors include the use of intra-aortic balloon pump (IABP), MV abnormalities, and concomitant MV replacement with a high‑profile bioprosthetic valve (Makhija et al. 2019; Huang 2024). Similarly, SAM has been reported after transcatheter aortic valve replacement (TAVR) (Weich et al. 2021; Ben-Dor et al. 2022).
SAM can occur in up to 13% of patients undergoing MVR (Ashikhmina et al. 2021), particularly in those with LV ejection fraction (LVEF) > 60%, excessive height of the MV leaflets (particularly of the posterior one), or who received complete ring annuloplasty (Loulmet et al. 2014). Moreover, bi-leaflet prolapse, a low ratio of the heights of the anterior to posterior MV leaflets, low end-systolic LV volume, and younger age at the time of surgery are reported as risk factors for SAM after MVR (Ashikhmina et al. 2021).
However, many reports of SAM in patients undergoing noncardiac surgery also exist. Luckner et al. (2005) reported the occurrence of severe perioperative hypotension due to SAM in three patients undergoing orthopaedic surgery for bilateral femoral neck fracture, cholecystectomy with hepatic resection for gallbladder carcinoma, and femur prosthesis for repeated hip prosthesis dislocations, respectively. None of the three patients had anatomical predisposing factors for SAM, while hypovolaemia and anaesthesia-induced vasodilation were regarded to as the main precipitating factors in all three cases. Other authors reported SAM during thoracoscopic surgery (Monaco et al. 2022), liver transplantation (Essandoh et al. 2016), kidney transplantation (complicated by anaphylaxis following antibiotic administration) (Nooli et al. 2023) and, in general, due to a decrease in preload and/or increase in heart rate secondarily to general or neuraxial anaesthesia (Chou 2022) or pneumoperitoneum (Fujita et al. 2015).
Diagnosis and Management
Unexpected severe hypotension is the most common manifestation of SAM in ICU or surgical patients (Luckner et al. 2005). However, hypoxaemia due to mitral regurgitation and pulmonary oedema has also been reported as a possible clinical presentation (Chou 2022; Fujita et al. 2015). Severe haemodynamic instability (particularly which worsens by administering or increasing the doses of inotropes), a progressive increase in norepinephrine requirements, and worsening oxygenation should prompt cardiac ultrasound examination in order to rapidly differentiate among cardiogenic shock due to impaired ventricular function, distributive shock, and LVOTO/SAM (Duncan et al. 2023).
Cardiac ultrasound examination
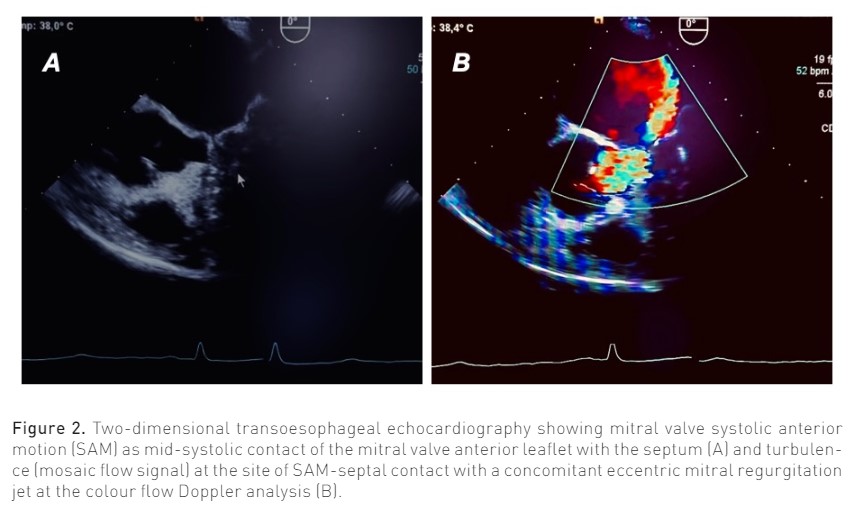
Main echocardiographic findings in patients with LVOTO/SAM include a crescent shaped LV cavity, normal or slightly supernormal LVEF, asymmetrical septal hypertrophy (ASH) with wall thickness >1.5 cm in basal anteroseptum (Cresci 2017); LVOTO is dynamic and variable with loading conditions, with an outflow gradient > 30 mmHg (velocity >2.7 sec); SAM is visible as the mid-systolic contact of the MV anterior leaflet with the septum (particularly in M-mode) (Guigui et al. 2022), while colour flow Doppler reveals turbulence (mosaic flow signal) at the site of SAM-septal contact (Raut et al. 2018) (Figure 2); in most severe cases, closure of the aortic valve due to reduced sub-valvular pressure can occur; MR secondary to SAM is characterised by an eccentric posteriorly directed jet due to failed apposition of mitral leaflets, unlike the central or anteriorly directed jet which is typical of primary MV disease (Guigui et al. 2022). Further descriptions of the anatomical/echocardiographic features of HCM are beyond the scope of the present review and can be found elsewhere (Guigui et al. 2022; Sherrid et al. 2016).
Prevention and Treatment
Medical management of SAM in HCM patients includes chronic therapy with beta-blockers and calcium antagonists (mostly verapamil) to improve LV filling and volume loading and avoid afterload reduction (Guigui et al. 2022; Maron et al. 2018).
Mavacamten, a myosin adenosine triphosphatase inhibitor, was shown to improve symptoms, exercise capacity, and LVOTO, as well as SAM and important measures of LV diastolic function and biomarkers of myocardial wall stress in a randomised controlled trial (RCT) of 251 patients with symptomatic (obstructive) HCM (Hegde et al. 2021; Olivotto et al. 2020). To our knowledge, this is the only RCT involving patients with SAM. In HCM patients with refractory obstruction and persistent symptoms despite medical therapy, surgical septal myectomy should be preferred, particularly in younger patients, while percutaneous alcohol septal ablation is a reasonable alternative in higher-risk patients; MV surgery is generally indicated for patients with mild to moderate septal hypertrophy, intrinsic MV disease, or papillary muscle abnormalities (Guigui et al. 2022; Affronti et al. 2021).
In patients with HCM or isolated (septal) hypertrophy, the anaesthetic strategy should be carefully evaluated. Although neuraxial anaesthesia was historically considered contraindicated, selective techniques which avoid excessive vasoplegia could probably be performed, taking the possibility of SAM into consideration and preventing hypovolaemia. The choice of the anaesthetic regimen can be particularly challenging in conditions in which general anaesthesia represents an issue, such as during pregnancy (Hussey et al. 2023). Anaesthesiologists should also consider peripheral nerve blockades over neuraxial anaesthesia to avoid vasodilation (Monaco et al. 2022).
In light of the pathophysiology discussed above, the cornerstones of acute treatment of severe shock due to SAM in the critical care or perioperative setting are fluid resuscitation, use of vasoconstrictors (pure vasoconstrictors such as phenylephrine or vasopressin may be preferable to norepinephrine), and discontinuation of inotropic (and vasodilating) agents; moreover, b-blockers and/or calcium channel blockers (verapamil) can be used to decrease heart rate and cardiac contractility (Dugar et al. 2016; Mingo et al. 2006; Abbas et al. 2019; Duncan et al. 2023; Makhija et al. 2019; Poveda-Jaramillo et al. 2018). Particularly in the setting of myocardial infarction, discontinuation of IABP mechanical support should also be considered (Harrington et al. 2023), while especially after cardiac surgery, atrial pacing can be useful as the conversion from normal sinus rhythm to junctional rhythm can worsen SAM, possibly due to the lack of the atrial kick (Seino et al. 2018).
Administration of vasopressin has been shown to significantly reduce norepinephrine requirements, improve haemodynamics, and reduce the severity of MR and pulmonary oedema in septic shock patients with SAM (Balik et al. 2020).
The ultra-short-acting selective b-blockers (esmolol, landiolol) should be preferred for the management of heart rate and arrhythmias in septic patients due to their safety and simplicity of use (Poveda-Jaramillo et al. 2018). In cardiac surgery, e.g. following MV repair, the lack of response to esmolol administration has been proposed as a strategy to identify patients with SAM who may be not responsive to conservative therapy, needing surgical revision (Poveda-Jaramillo et al. 2018; Landoni et al. 2011).
Conclusion
Although usually associated to the setting of HCM and cardiac surgery, SAM can also occur in the absence of pre-existing heart disease, especially in association with the haemodynamic changes which often occur in the perioperative and critical care setting, such as hypovolaemia, vasoplegia, increased heart contractility, and tachycardia. Accordingly, all intensivists and anaesthesiologists should be aware of the pathophysiology, risk factors, and correct management of SAM and actively seek it out (with echocardiography), particularly in the presence of severe haemodynamic instability which worsens with the increase in inotropic drug doses. This condition can mimic fluid overload and cardiogenic shock, and treating it with diuretics, vasodilators, and inotropic drugs leads to a progressive worsening in haemodynamics, hypoxaemia and shock or sudden death.
Conflict of Interest
None.
References:
Abbas H et al. (2019) Transient Systolic Anterior Motion of the Anterior Mitral Valve Leaflet in a Critical Care Patient with a Structurally Normal Heart. Cureus. 11(1):e3963.
Affronti A et al. (2021) Surgery for Hypertrophic Obstructive Cardiomyopathy: Comprehensive LVOT Management beyond Septal Myectomy. J Clin Med. 10(19):4397.
Alhaj EK et al. (2013) Symptomatic exercise-induced left ventricular outflow tract obstruction without left ventricular hypertrophy. J Am Soc Echocardiogr. 26(5):556-65.
Ashikhmina E et al. (2021) Risk factors and progression of systolic anterior motion after mitral valve repair. J Thorac Cardiovasc Surg. 162(2):567-577.
Balik M et al. (2020) Vasopressin in Patients with Septic Shock and Dynamic Left Ventricular Outflow Tract Obstruction. Cardiovasc Drug Ther. 34(5):685-688.
Baulier C et al. (2022) Left Ventricular Outflow Tract Obstruction in Patients Treated With Milrinone for Cerebral Vasospasm: Case Report and Literature Review. JMIRx Med. 3(2):e31019.
Ben-Dor I et al. (2022) A Word of Caution Before Treating Aortic Stenosis in Patients With Concomitant LVOT Obstruction. JACC Case Rep. 4(18):1162-1168.
Chauvet JL et al. (2015) Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care. 19(1):262.
Chou CJ (2022) Unexpected systolic anterior motion of the mitral valve-related hypoxemia during transurethral resection of the prostate under spinal anesthesia: a case report. BMC Anesthesiol. 22(1):207.
Conradi PM et al. (2021) Dynamic left ventricular outflow tract obstruction in Takotsubo cardiomyopathy resulting in cardiogenic shock. BMJ Case Rep. 14(3):e240010.
Cresci S (2017) Hypertrophic Cardiomyopathy. In: Nishad Quader. The Washington Manual of Echocardiography 2nd Edition. Wolters Kluwer. pp. 103-117.
Di Vece D et al. (2021) Dynamic Left Intraventricular Obstruction Phenotype in Takotsubo Syndrome. J Clin Med. 10(15):3235.
Dugar S et al. (2016) All Shock States are not the same. Systolic Anterior Motion of Mitral Valve Causing Left Ventricular Outflow Tract Obstruction in Septic Shock. Ann M Thorac Soc. 13(10):1851-1855.
Duncan CF et al. (2023) Mitral regurgitation in the critically ill: the devil is in detail. Ann Intensive Care. 13(1):67.
Essandoh M et al. (2016) Refractory Hypotension after Liver Allograft Reperfusion: A Case of Dynamic Left Ventricular Outflow Tract Obstruction. Front Med (Lausanne). 16:3:3.
Evans L et al. (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 49(11):e1063-e1143.
Fujita Y et al. (2015) Sudden hypoxemia after uneventful laparoscopic cholecystectomy: another form of SAM presentation. BMC Anesthesiol. 15:51.
Guigui SA et al. (2022) Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: a narrative review. J Thorac Dis. 14(6):2309-2325.
Harrington J et al. (2023) The Role of Focused 2-Dimensional Echocardiography in Managing Left Ventricular Outflow Tract Obstruction Mimicking Cardiogenic Shock. J Intensive Care Med. 38(10):897-902.
Hegde SM et al. (2021) Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients With Obstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 78(25):2518-2532.
Huang K (2024) Reversibility systolic anterior motion of the mitral valve after aortic valve replacement for severe aortic stenosis. Clin Case Rep. 12(1):e8416.
Hussey PT et al. (2023) A Case Report of Spinal Anesthesia for Cerclage Placement in the Setting of Severe Hypertrophic Obstructive Cardiomyopathy. AA Pract. 17(4):e01675.
Landoni G et al. (2011) Validation of a decision-making strategy for systolic anterior motion following mitral valve repair. Ann Card Anaesth. 14(2): 85-90.
Levine RA et al. (2014) Diastolic leading to systolic anterior motion: new technology reveals physiology. J Am Coll Cardiol. 64:1996-9.
Loulmet D et al. (2014) Systolic anterior motion of the mitral valve: a 30-year perspective. J Thorac Cardiovasc Surg. 148(6):2787-93.
Luckner G et al. (2005) Systolic anterior motion of the mitral valve with left ventricular outflow tract obstruction: three cases of acute perioperative hypotension in noncardiac surgery. Anesth Analg. 100(6):1594-1598.
Makhija N et al. (2019) Left ventricular outflow tract obstruction following aortic valve replacement: A review of risk factors, mechanism, and management. Ann Card Anaesth. 22(1):1-5.
Maron BJ et al. (2018) Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. J Am Coll Cardiol HF. 6:353–63.
Mingo S et al. (2006) Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol. 112(3):393-396.
Monaco F et al. (2022) Mitral Valve Systolic Anterior Motion in Robotic Thoracic Surgery as the Cause of Unexplained Hemodynamic Shock: From a Case Report to Recommendations. J Clin Med. 11(20):6044.
Nooli NP et al. (2023) Role of Rescue Transesophageal Echocardiography During Intraoperative Anaphylaxis Complicated by Dynamic Left Ventricular Outflow Tract Obstruction. Cardiothorac Vasc Anesth. 37(4):565-569.
Olivotto I et al. (2020) Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 396(10253):759-769.
Pisano A (2021) Continuity Equation and Bernoulli’s Theorem: Airplanes, Venturi Masks, and Other Interesting Things (for Anesthesiologists and Intensivists). In: Pisano A. Physics for Anesthesiologists and Intensivists: From Daily Life to Clinical Practice, 2nd Edition. Cham, Switzerland: Springer. pp. 77-88.
Poveda-Jaramillo R et al. (2018) Ultra-short acting b-blockers (esmolol and landiolol) in the perioperative period and in critically ill patients. J Cardiothorac Vasc Anesth. 32(3):1415-1425.
Raut M et al. (2018) Awareness of 'Systolic Anterior Motion' in Different Conditions. Clin Med Insights Cardiol. 12:1-2.
Ro R et al. (2014) Vector flow mapping in obstructive hypertrophic cardiomyopathy to assess the relationship of early systolic left ventricular flow and the mitral valve. J Am Coll Cardiol. 64:1984-95.
Seino Y et al. (2018) Transient systolic anterior motion with junctional rhythm after mitral valve repair in the intensive care unit. Crit Ultrasound J. 10(1):30.
Sherrid MV et al. (2016). The mitral valve in obstructive hypertrophic cardiomyopathy: a test in context. L Am Coll Cardiol. 67 (15):1846-1858.
Slama M et al. (2016) Left ventricular outflow tract obstruction in ICU patients. Curr Opin Crit Care. 22(3):260-6.
Uematsu S et al. (2017) Clinical features of the systolic anterior motion of the mitral valve among patients without hypertrophic cardiomyopathy. J Cardiol. 69(2):495-500.
Weich HSV et al. (2021) Dynamic Left Ventricular Outflow Tract Obstruction Post-Transcatheter Aortic Valve Replacement. JACC Case Rep. 3(6):871-874.
Yasuhiro H et al. (2022) Dynamic changes in the three-dimensional mitral complex geometry in a case of takotsubo cardiomyopathy with transient systolic anterior movement of the mitral valve. J Cardiol Cases. 26:190–193.