ICU Management & Practice, Volume 24 - Issue 3, 2024
Ultrasound serves as a tool to enhance diagnostic precision for decision-making in life-threatening situations in the ICU. This article will delve into the evidence of ultrasound monitoring across different scenarios.
Introduction
In the contemporary landscape of medical diagnostics, ultrasonography stands out as a pivotal instrument, offering a non-invasive, cost-effective, and readily accessible modality for the real-time visualisation of internal anatomical structures. Its significance extends beyond mere imaging, serving as a critical adjunct in the decision-making process across various medical disciplines.
Ultrasonography's versatility is showcased through its application in diverse clinical scenarios, ranging from emergency medicine to chronic disease management. Moreover, ultrasonography's diagnostic precision is enhanced by the continuous advancements in ultrasound technology, including high-resolution imaging and Doppler capabilities.
Importantly, the real-time nature of ultrasonography permits a dynamic assessment of physiological functions, a feature unattainable by other imaging modalities.
Neurocritical Patient
Neuromonitoring is vital in the detailed management of individuals with traumatic brain injuries (TBI), enabling the swift detection of issues like increased intracranial pressure (ICP) (Martinez-Palacios et al. 2023). The diagnosis and treatment of ICP reduces morbidity and mortality (Chen et al. 2023).
The gold standard monitoring ICP necessitates the insertion of an invasive transducer into the parenchymal tissue or the brain ventricle, carrying potential risks of complications such as haemorrhage and infection (Raboel et al. 2012).
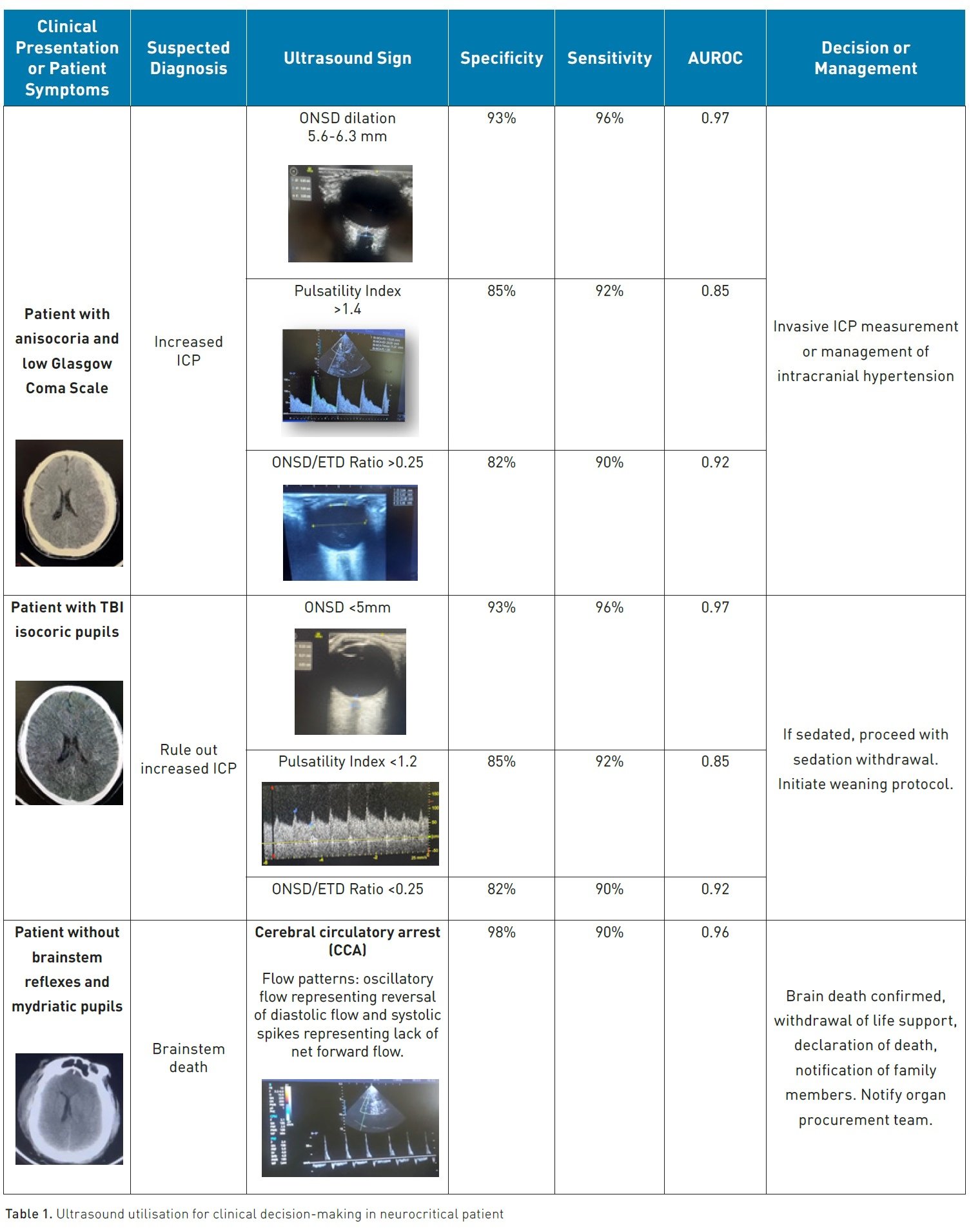
Ultrasound has become a preferred method for gauging the optic nerve sheath diameter (ONSD) owing to its convenience, functionality, safety, consistency, and lack of ionising radiation exposure or recognised adverse reactions (Montgomery et al. 2023).
The optic nerve is enveloped by a sheath derived from the meninges, stretching towards the orbit. This linkage allows for the movement of cerebrospinal fluid (CSF), thereby permitting analogous pressure shifts within the intracranial and orbital subarachnoid spaces. As a result, employing ultrasound to detect raised ICP via ONSD assessments is increasingly accepted in trauma, neurosurgical, and emergency medical settings (Fernando et al. 2019)
The fundamental procedures for measuring the ONSD via ocular ultrasound are outlined as follows:
- Position the patient in a supine manner.
- Apply gel to the closed upper eyelid, then place the high-frequency linear probe on it. This probe enhances the contrast between the nerve and the retrobulbar fat.
- Manipulate the probe to visualise the entrance of the optic nerve into the globe.
- The ONSD should ideally be measured 3mm posterior to the eye globe. The intersection point of the optic nerve and the ophthalmic artery is also a recommended site for measurement.
- Obtain multiple readings for each eye and calculate the average to minimise the risk of variance (Richards et al. 2023).
Systematic review and meta-analysis showed a mean ONSD in the included studies of 5.82 mm (Monrtofano et al. 2021). ICP can be calculated using the formula ONSD = 5.69×ONSD –8.23 mmHg.
To enhance the reliability of ONSD measurement, the introduction of the ONSD to eyeball transverse diameter (ETD) ratio was proposed (Vaiman et al. 2014). The transverse diameter of the eyeball and the sheath is measured and divided by this measurement; if it results in a value greater than 0.25, it indicates ICP (Du et al. 2019).
Transcranial Doppler (TCD) and transcranial colour-coded duplex sonography (TCCS) serve as essential real-time neurological monitoring instruments within the Intensive Care Unit (ICU). TCD ultrasonography can be conducted bedside to ascertain and monitor cerebral blood flow (CBF), gauged by mean blood-flow velocity (MFV), and ICP can be determined through Pulsatility Index (PI) values of the middle cerebral artery (MCA), as well as other major intracranial vessels, irrespective of the patient's level of consciousness or sedation status (Fatima et al. 2019). The PI stands out as a critical haemodynamic parameter offered by TCD/TCCS (Pinillos et al. 2021)
Patients with TBI who underwent diagnostic TCD monitoring for increased ICP or vasospasm (VSP) and exhibited abnormal TCD findings (mean flow velocity [MFV] >120 cm/s or MFV <35 cm/s, PI >1.2) were more than three times as likely to experience a poor outcome compared to patients with TBI and normal TCD monitoring (Fatima 2019) The odds ratio (OR) for poor outcome in these cases was 3.87, with a 95% confidence interval (CI) ranging from 2.97 to 5.04, indicating a significant association (P < 0.00001)
It's important to note that despite its prognostic value, the use of TCD as a diagnostic tool for intracranial hypertension is not considered standard practice according to Robba and Taccone (2019) and not solely dependent on the PI > 1.4 because elevated pulse pressure, hypercapnia, hypocapnia, bradycardia, patient age, insonation angle, hypothermia, and hyperthermia can all influence the results of TCD examinations.
Brain Death
TCD is routinely employed as an ancillary test to confirm the absence of CBF. The identification of specific TCD patterns is crucial for determining cerebral circulatory arrest (CCA): these patterns include reverberating flow, systolic spikes, and the disappearance of previously recorded flow velocities (FV). The presence of the mentioned flow patterns across all major intracranial vessels is required to confirm brain death (Robba and Taccone 2019).
In a meta-analysis synthesis of 22 RCT, TCD was found a sensitivity of 90% and a specificity of 98%, when benchmarked against the gold standard (Chang et al. 2016).
Cardiac POCUS (FoCUS: Focused Cardiac Ultrasound)
One of the most sensitive (S) and specific € findings when scanning the heart is pericardial effusion (S:96%, E:98%), which, in the right context and with certain characteristics, becomes an absolute indication for immediate pericardiocentesis (Lau and See 2022).
The context would be a patient in a state of obstructive shock who, upon standard cardiac scanning (parasternal long and short axis, apical 4 chambers and/or subcostal), displays an anechoic image that separates the pericardial layers by at least 10 mm. If this distance is smaller, pericardiocentesis is contraindicated as it is considered a small effusion with little probability of being responsible for the state of shock, and with technical complexity for performing pericardiocentesis, the risk being greater than the potential benefit (Imazio and De Ferrari 2020). The echocardiographic signs we can find in cardiac tamponade are right atrial diastolic collapse (S: 95-100%, E: 70-80%), right ventricular diastolic collapse (S: 90-95%, E: 95-100%), respiratory cycle changes in the E wave velocity of the mitral flow > 25% and of the (Hamzaoui et al. 2013) E wave in the tricuspid flow > 40%, the last two signs are surrogates of the paradoxical pulse (S: 98%, E: 83%), dilation of the IVC (inferior vena cava) > 20 mm with a reduction in its variability (<50%) with the respiratory cycle (S: 95-100%, E: 40-50%).
Another immediate attention pathology is pulmonary thromboembolism (PTE), traditionally only diagnosed with pulmonary angiography or perfusion scintigraphy. However, since 2019, the European cardiology guidelines for PTE established echocardiographic signs that could aid in the diagnosis of high-risk PTE and make the decision for thrombolysis without waiting for angiography, as this study is not available in many hospital centres (Konstantinides et al. 2020). One of the most used signs of PTE is the growth of the right ventricle (RV) compared to the left ventricle (LV) with an RV/LV ratio > 1 (S: 50%, E: 93%). The D sign, referring to the abnormal flattening or bulging of the septal wall towards the LV due to RV pressure overload, has been reported with low sensitivity but high specificity for tamponade (S:29.7%, E: 96.2%). McConnell’s sign, which includes akinesia of the RV-free wall with abnormal RV apical hyperkinesis due to anchoring with the LV fibres in a hyperdynamic state, has an estimated sensitivity of 29.1% and specificity of more than 98%. The combination of a reduction in TAPSE (tricuspid annular plane systolic excursion) <17 mm and the 60/60 sign (pulmonary valve acceleration time <60 ms and tricuspid regurgitation gradient <60 mmHg) have a sensitivity of 51% and specificity of 86% (Falster et al. 2022). Early systolic notching in the Doppler study of the pulmonary artery flow has been reported with a sensitivity of up to 97% and specificity of 99% with an area under the curve of 0.96 (Bigdelu et al. 2023).
Another indispensable evaluation in echocardiography is the determination of heart failure, as if established, it can have significant implications in the approach to a patient, generally to decide the initiation of inotropics or to halt fluid resuscitation.
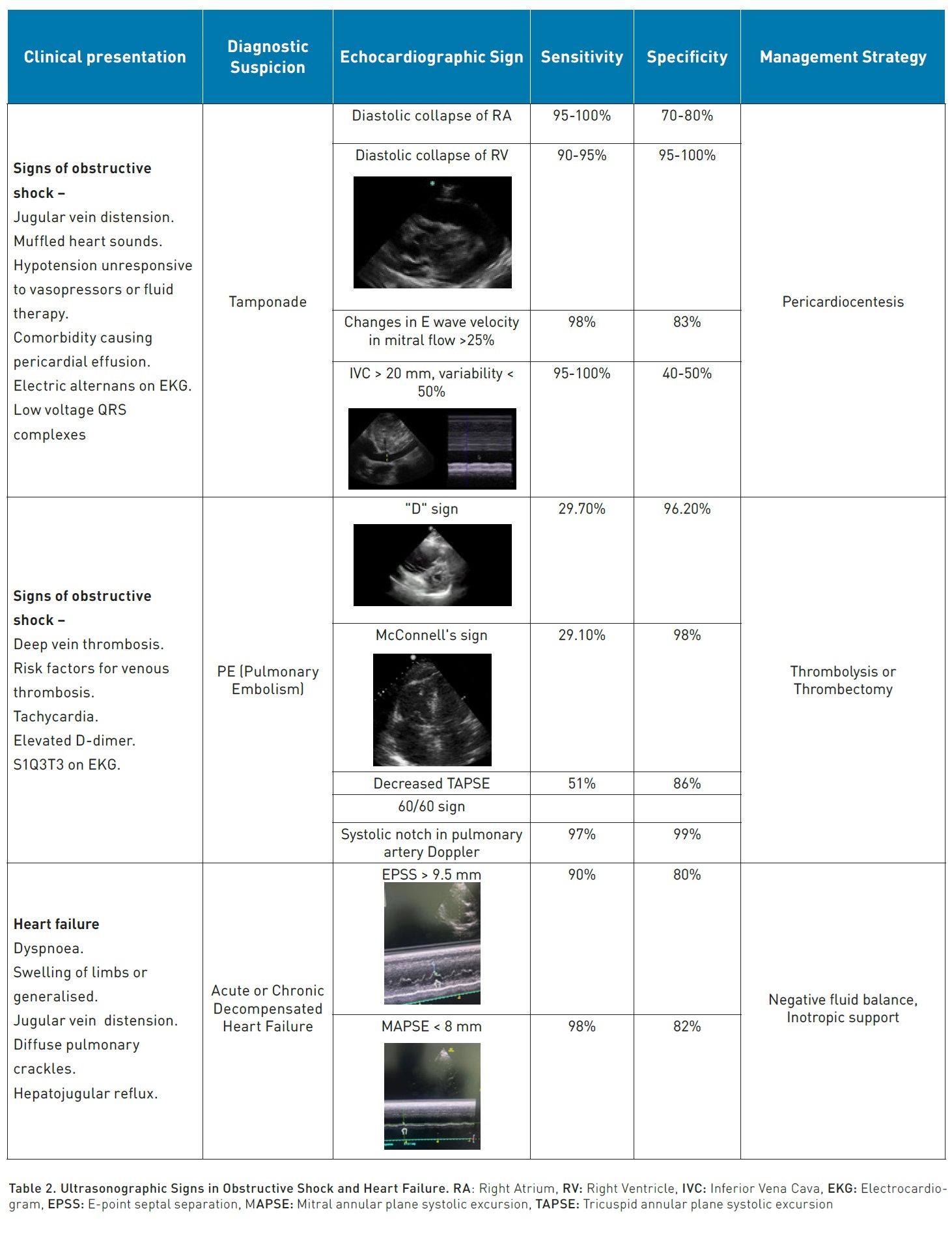
E-point septal separation (EPSS) has a strong correlation with LVEF (left ventricular ejection fraction), traditionally the cut-off point to define that the LVEF is below 40% is >7 mm (McKaigney 2014); however, a cut-off of 9.5 mm has been recently proposed to establish an LVEF <40% with an S: 90%, E: 80% and an area under the curve of 0.91 (Núñez-Ramos et al. 2022).
MAPSE (Mitral annular plane systolic excursion) allows for the evaluation of longitudinal shortening of the LV; a measure of less than 8 mm is associated with an LVEF < 50% with an S 98% and E 82%, while a value of more than 10 mm (Schick et al. 2022) has an S 90% and E 87% for preserved systolic function (>55%).
In general, POCUS has high sensitivity and specificity in identifying the type of shock presented by patients. From 0.77 for distributive shock to 0.93 for hypovolaemic shock, with specificity ranges going from 0.92 for hypovolaemic to 0.97 for obstructive shock (Yoshida et al. 2023).
Lung Ultrasound
This can reduce imaging techniques involving radiation and decrease the taking of radiographs in critical areas by up to 50% (Brogi et al. 2017). The lung exploration can be done with any transducer on a simple ultrasound machine, dividing the thorax into 4 quadrants per lung and using both two-dimensional and M modes. Subsequently, it is necessary to describe the pleural line and the artefacts that originate from this line for the diagnosis of the different clinical scenarios (Demi et al. 2023).
Acute Respiratory Failure
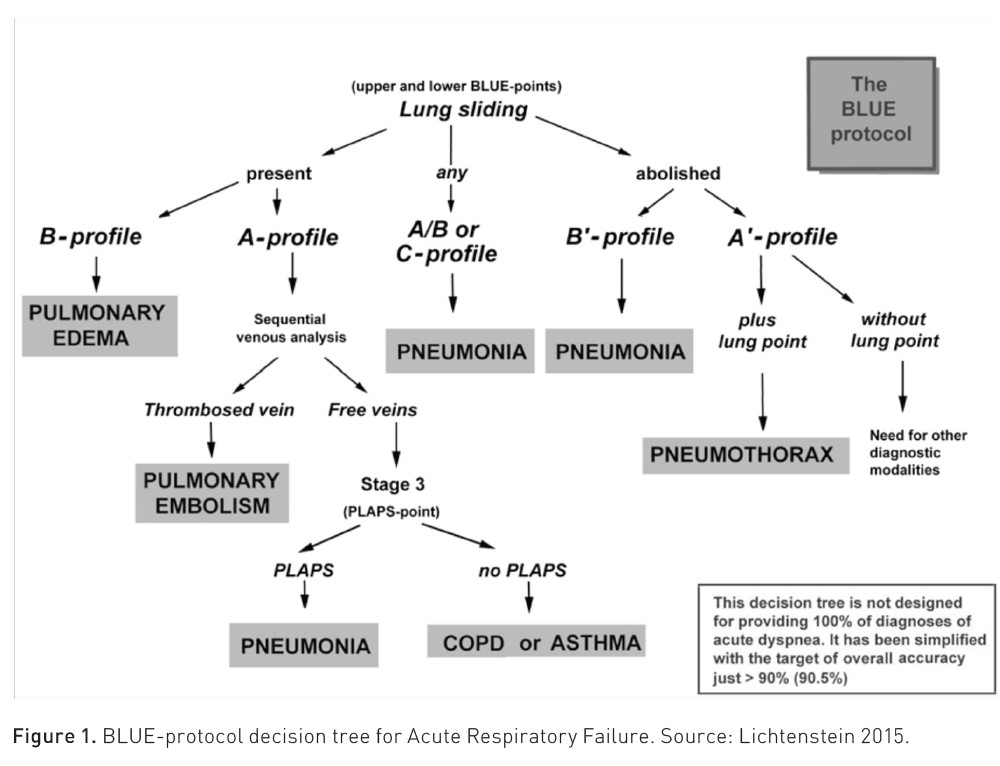
Lung ultrasound is sensitive to changes in lung aeration and density. Therefore, the increase in extravascular lung water, loss of aeration, or the combination of these phenomena modify the visualised image. In a meta-analysis with 1,232 patients, lung ultrasound had a sensitivity of 92 and a specificity of 98% for diagnosing the cause of acute respiratory failure. Pleural effusion and acute interstitial syndrome are the aetiologies most likely to be identified by lung ultrasound, reporting a pooled sensitivity of 95% (Yuan et al. 2021). Due to this diagnostic precision, international scientific societies issue strong recommendations for the use of lung ultrasound for the diagnosis of acute respiratory distress syndrome (ARDS), for the evaluation and classification of pulmonary oedema in heart failure, and for the evaluation of pleural diseases (Gargani et al. 2023).
Pneumothorax
Lung ultrasound presents 95% specificity for the diagnosis of pneumothorax when absence of pleural sliding and the barcode sign (also called stratosphere sign) are observed, and 100% specificity when the "lung point" sign is found respectively. The sensitivity is 90 to 95%, aiding in diagnosis when there is high clinical suspicion in patients with decreased mobility of a hemithorax, absence of respiratory sounds, hyperresonance on percussion, and some suspected cause (e.g., chest trauma, central venous access placement).
Pleural Effusion
In cases of pleural effusion syndrome, clinical presentation often includes observable signs such as decreased lung expansion on the affected side, diminished palpation and percussion, and reduced tactile fremitus. However, these clinical indicators exhibit low sensitivity and specificity. Utilising ultrasound as a diagnostic tool significantly enhances accuracy. Ultrasound findings typically reveal an intrapleural anechoic zone, commonly referred to as the sinusoid sign in M mode. The sensitivity and specificity of ultrasound in detecting pleural effusion range between an impressive 95% and 99%, respectively. This underscores the invaluable role of ultrasound in accurately diagnosing pleural effusion syndrome and guiding appropriate clinical management. Moreover, ultrasound also enables clinicians to perform thoracentesis, facilitating the safe and precise extraction of pleural fluid for diagnostic and therapeutic purposes.
Consolidation
In the case of consolidation syndrome or pulmonary condensation, characterised by systemic inflammatory response data (fever, tachycardia) and respiratory failure (dyspnoea and low oxygen saturation) which are not very sensitive and specific, together with more sensitive clinical data such as productive cough with purulent expectoration, lung ultrasound complements these findings with a sensitivity reaching 89% and specificity up to 97% when finding an irregular or fragmented pleural line accompanied by subpleural hyperechoic echoes, known as the shred sign, in addition to dynamic air bronchograms observed as branching intraparenchymal hyperechoic images, making this tool superior to clinical diagnosis of this syndrome.
Interstitial Syndrome
The interstitial alveolar syndrome could hardly be differentiated clinically from another cause of acute respiratory failure if not for the findings on lung ultrasound, where we will find three or more vertical hyperechoic artefacts, which originate vertically from the pleural line in the form of comet tails, and erase the A-lines, it is necessary for these findings to be found in two different insonation zones, these artefacts are known as B-lines and have a sensitivity of 95% and specificity of 91% for the diagnosis of this syndrome.
Diaphragmatic Dysfunction Myotrauma Associated with Mechanical Ventilation
Diaphragmatic Excursion: With a low-frequency transducer positioned longitudinally with the cephalic mark in the right subcostal region between the anterior axillary line and the midclavicular line, the posterior third of the right hemidiaphragm can be visualised in two-dimensional mode. During inspiration, there is a caudal displacement (excursion) that can be explored in M mode with the exploration line positioned perpendicular to the diaphragm. Absent or reduced excursion <10 mm during a spontaneous breathing trial indicates diaphragmatic dysfunction.
Diaphragmatic Thickening: With a high-frequency transducer perpendicular to the lateral thoracic wall in the mid-axillary intercostal region at the zone of apposition (between the 9th and 10th intercostal spaces), the diaphragm is identified 2 – 4 cm from the skin as a three-layer structure; an internal hypoechoic muscular layer surrounded by two hyperechoic external membranes (the peritoneum and the pleura). The thickness of the muscle at the end of expiration (at rest) and the thickening and stiffness of the diaphragm during inspiration should be analysed in two-dimensional or M mode. The lower limit for the normal thickness of the diaphragm in healthy individuals is 1.5 mm (Santana et al. 2023). Values lower than this can predict diaphragmatic atrophy. Also, a decrease of >10% in serial measurements of the diaphragm suggests atrophy (Goligher et al. 2015). The diaphragm thickening fraction is defined as the percentage change in the thickness of the diaphragm during inspiration. It represents the inspiratory effort of the diaphragm, and a value < 20% is useful for the diagnosis of diaphragmatic dysfunction (Inspiratory Diameter – Expiratory Diameter)/Expiratory Diameter)X100. In a recent meta-analysis, the diaphragmatic excursion has an S 80% and E 80%, and the thickening fraction an S 85% and E 75%; therefore, they have acceptable diagnostic accuracy as predictors of success for the withdrawal of mechanical ventilation (Parada-Gereda et al. 2023).
Focused Assessment With Sonography in Trauma: ECO-FAST
Trauma represents a significant cause of mortality in young individuals (Savoia et al. 2023). Closed abdominal injuries (solid organ injuries such as the liver or spleen, mesenteric and visceral tears) can cause significant bleeding and haemodynamic instability, with hypovolaemic shock being one of the main causes of trauma-related mortality (Stengel 2015).
According to the protocols established by Advanced Trauma Life Support (ATLS), the first step as a standard, quick, repeatable, economical, and reliable diagnostic tool for the early detection of intra-abdominal haemorrhage in patients with severe injuries is the performance of an abdominal ultrasound (Chaijareenont et al. 2020) or a focused assessment with sonography in trauma (FAST).
The FAST protocol is based on the principle that free fluid (FF) such as blood can accumulate in certain anatomical locations in the supine patient, its main objectives being to identify FF and at the same time guide decision-making in the resuscitation stage, mainly in the polytraumatised patient (Gallardo et al. 2023).
Whom, where, and when to apply the fast or e-fast protocol?
There are three important indications for performing the FAST protocol:
- Closed abdominal trauma with haemodynamic instability.
- Penetrating trauma in the thoracoabdominal transition, where there is doubt about penetration into the abdominal cavity, with haemodynamic instability.
- Haemodynamic instability of unknown cause (Savoia et al. 2023).
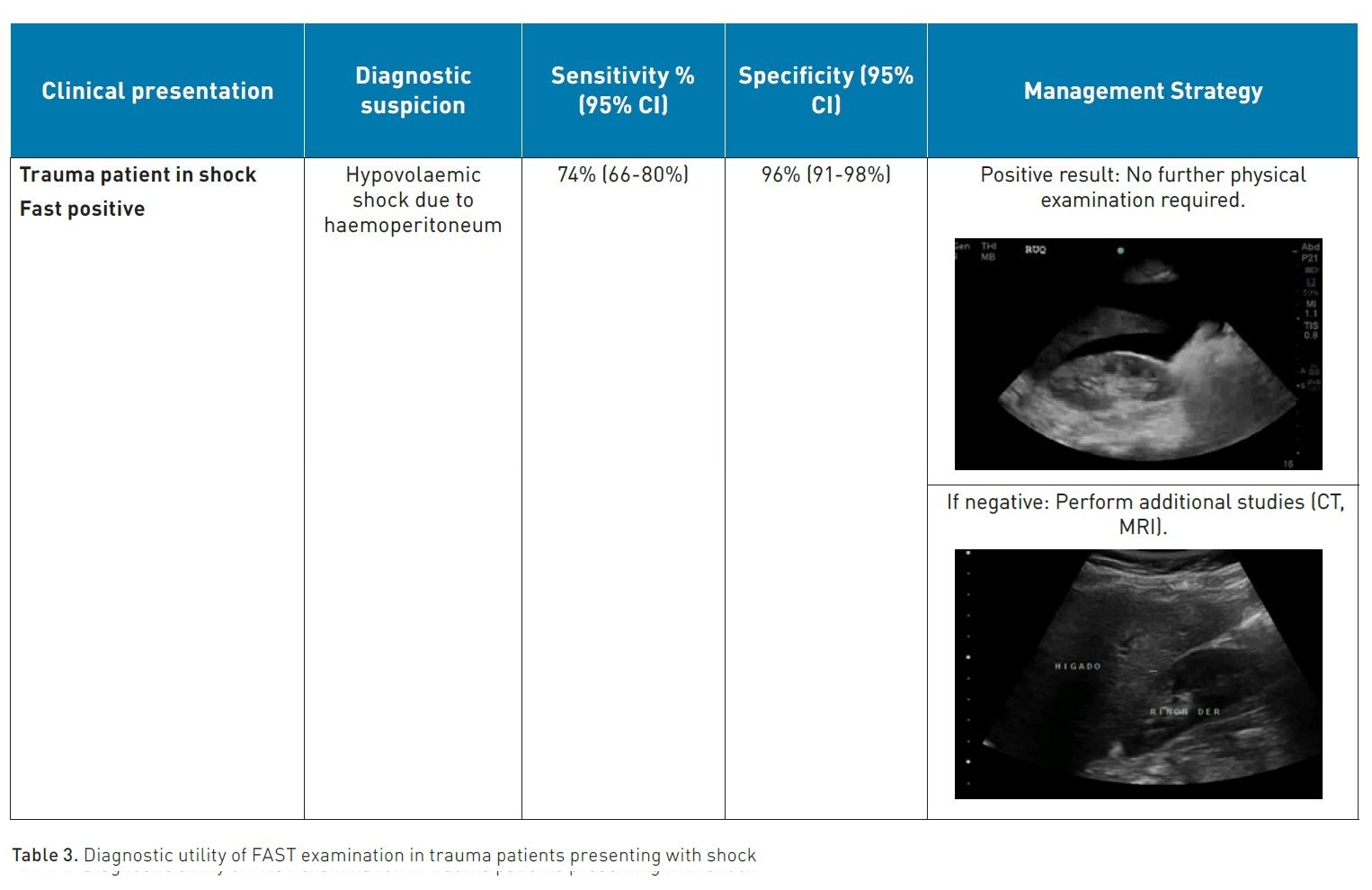
How to perform the fast and e-fast protocol
This protocol should be carried out at the patient's bedside, avoiding their transfer to a distant cabinet unit. Likewise, it should be applied during the evaluation of circulation within the ATLS protocol's ABCDE algorithm to address the presence of free fluid and, if applicable, the possible causes of cardiac tamponade (Savoia et al. 2023).
In the thorax, FF can be found in the pericardial and pleural spaces; if the antero-superior thorax is added, it is called E-FAST or extended FAST, useful for detecting the presence of pneumothorax. It is performed using the phased array transducer (2-4 MHz) or the convex transducer (2-5 MHz) to obtain images of the right and left upper quadrants and the suprapubic regions (Zhou and Wiley 2024). In the abdomen, the dependent spaces are susceptible to accumulating FF, such as the Morrison's space (located between the liver and the right kidney), the splenorenal space (between the spleen and the left kidney), above the spleen (subphrenic space) and within the pelvis, FF will accumulate in the pouch of Douglas. Nishijima et al. conducted a study in 2012 with the objective of systematically evaluating the accuracy and precision of symptoms, signs, laboratory tests, and bedside imaging studies for identifying intra-abdominal injuries in patients with diffuse abdominal trauma. Studies examining the identification of intra-abdominal injuries (12 studies) and a separate search for studies evaluating bedside ultrasound (22 studies) were included. It was found that the presence of intraperitoneal fluid or organ injury on bedside ultrasound evaluation is more accurate than any anamnesis and physical examination findings (LR, 30; 95% CI, 20-46%). On the other hand, in 2018, Stengel et al. conducted a Cochrane review of retrospective and prospective studies, including 34 studies with a total of 8635 patients, evaluating the diagnostic accuracy of FAST for thoracoabdominal injuries in patients with closed trauma, taking as reference standard Computed Tomography, Magnetic Resonance, laparotomy, thoracotomy or autopsy.
The primary outcome was the diagnosis of any thoracoabdominal injury; defined as free abdominal or thoracic fluid, retroperitoneum, pericardium or mediastinum, organ injury (spleen laceration, or another solid organ) as well as vascular injury (aortic dissection or injury to other vessels) and other injuries.
The results indicate that positive findings from point-of-care ultrasound can guide treatment decisions with a specificity of 0.96 and can contribute to reducing the need for imaging during trauma evaluation, especially in cases of thoracic trauma. However, for patients with abdominal and paediatric trauma, negative results from point-of-care ultrasound do not rule out injuries (with a sensitivity of 0.68 and 0.63, respectively); therefore, patients with negative results from point-of-care ultrasound require further evaluation to detect injuries (Long and April 2019).
Ultrasound for Evaluation of Thrombosis in Pelvic Limbs
Deep vein thrombosis (DVT) is part of the clinical spectrum of venous thromboembolic disease (VTE), with an incidence estimated at 1-2 episodes per 1000 people, constituting the 3rd cause of cardiovascular mortality in developed countries (Muñoz 2020).
The genesis of venous thromboembolism is multifactorial, requiring the presence of predisposing and/or triggering factors for its development. A prothrombotic and proinflammatory aetiology has been proposed, where coagulation factors interact with immune system cells (Khan et al. 2021). Triggering factors are associated with Virchow's Triad (venous stasis, endothelial injury, and hypercoagulability). Tissue injury leads to endothelium activation, which ultimately activates factor XII, contributing to thrombus formation (Zamarrón et al. 2021). It is crucial to achieve an accurate diagnosis of deep vein thrombosis (DVT) to prevent acute complications, such as pulmonary embolism, as well as chronic ones associated with post-thrombotic syndrome.
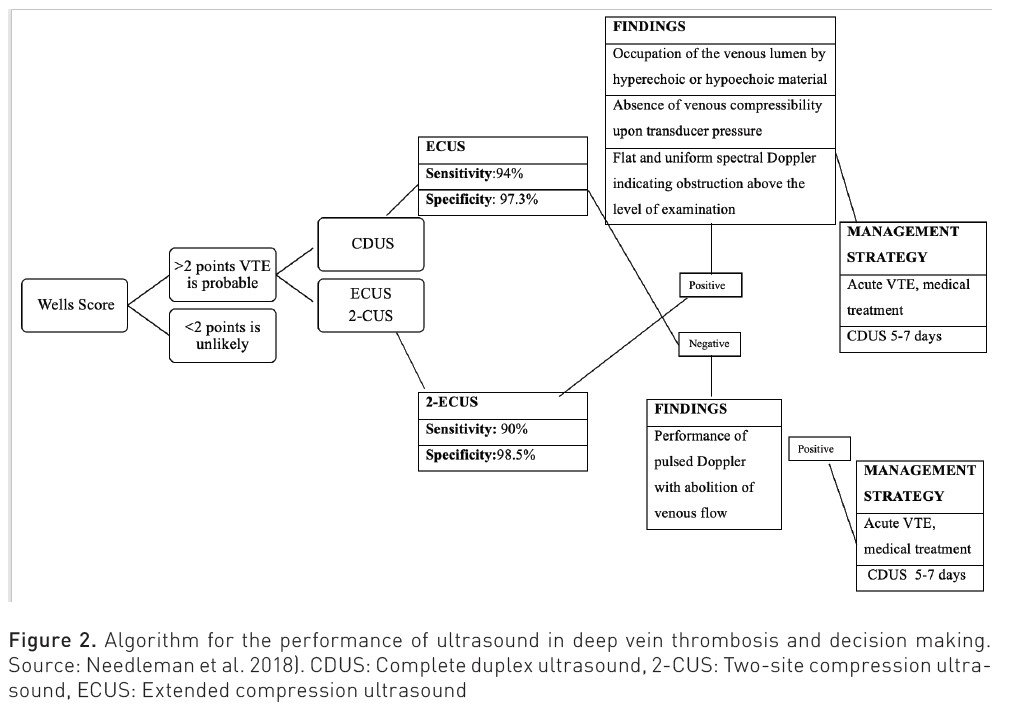
Diagnostic algorithms for DVT have been developed that incorporate clinical probability models based on the patient's medical history, D-dimer levels, and imaging tests. Among these tests, venous compression ultrasonography stands out as the preferred technique due to its non-invasive nature, ease of performance, and the ability to be repeated if necessary. The basic venous ultrasound examination is carried out using a linear transducer emitting medium to high frequencies (7-12 MHz). This allows for the assessment of both the femoropopliteal and distal axes, as well as the saphenous axes. Certain venous sectors may require the use of transducers with greater penetration: curvilinear transducers of low to medium frequencies (3-5 MHz), which are used to assess the iliac venous axis (Martí et al. 2023).
Five ultrasound signs that should be evaluated to diagnose venous thrombosis of the limbs are described (Kakkos et al. 2021):
- Confirmation in B mode or grey scale of the venous lumen occupied by hyperechoic or hypoechoic material.
- Occupation of the venous lumen by thrombotic material, leading to the most specific sign of venous thrombosis; absence of venous compressibility upon pressure with the transducer. This lack of compressibility can also be due, although without the evidence of the occupied venous lumen, to the venous plethora that may be created by obstructions proximal to the explored venous segment.
- Venous flow is abolished in Doppler mode as in pulsed Doppler.
- The absence of modulation or respiratory ease in the evaluation of flow at a venous point can translate into obstruction of the venous sector between the right atrium and the insonated vein.
- The absence of an A wave increases with muscular compression in the insonated sector.
Bhatt M et al. (2020) conducted a systematic review and meta-analysis to assess the accuracy of diagnostic tests for DVT of the lower extremities in both first-time and recurrent episodes, including proximal compression ultrasound (US), whole-leg ultrasound, serial US, and quantitative high-sensitivity D-dimer assays. The review included 43 studies. For any suspected DVT, the pooled estimates of sensitivity and specificity for proximal compression ultrasound were 90.1% (95% confidence interval [CI], 86.5-92.8) and 98.5% (95% CI, 97.6-99.1), respectively. For whole-leg ultrasound, the pooled estimates were 94.0% (95% CI, 91.3-95.9) and 97.3% (95% CI, 94.8-98.6); for serial ultrasound, the pooled estimates were 97.9% (95% CI, 96.0-98.9) and 99.8% (95% CI, 99.3-99.9). The pre-test probability of DVT, often assessed by a clinical decision rule, will influence how, along with the sensitivity and specificity estimates, patients will be managed.
Conclusion
Ultrasonography proves itself as a pivotal tool in modern medical diagnostics, marked by its versatility across various disciplines. Its non-invasive, cost-effective nature, coupled with real-time imaging capabilities, makes it indispensable in settings ranging from neurocritical care to cardiology and emergency medicine. The technology's evolution continues to enhance diagnostic accuracy and safety, significantly impacting patient management and care outcomes. As ultrasound technology advances, its integration into clinical practice is expected to deepen, underscoring the need for ongoing training in ultrasonographic techniques to fully leverage this potential.
Conflict of Interest
None.
References:
2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal. 36(42):2921-2964.
Bhatt M, Braun C, Patel P et al. (2020) Diagnosis of deep vein thrombosis of the lower extremity: a systematic review and meta-analysis of test accuracy. Blood Advances 4(7):1250-64.
Bigdelu L, Daloee MH, Emadzadeh M et al. (2023) Comparison of echocardiographic pulmonary flow Doppler markers in patients with massive or submassive acute pulmonary embolism and control group: A cross-sectional study. Health Sci Rep. 6(5):e1249.
Brogi E, Bignami E, Sidoti A et al. (2017) Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound. 15(1):23.
Chaijareenont C, Krutsri C, Sumpritpradit P et al. (2020) FAST accuracy in major pelvic fractures for decision-making of abdominal exploration: Systematic review and meta-analysis. Annals of medicine and surgery. 60:175-181.
Chang JJ, Tsivgoulis G, Katsanos AH et al. (2016) Diagnostic Accuracy of Transcranial Doppler for Brain Death Confirmation: Systematic Review and Meta-Analysis. Am J Neuroradiol. 37(3):408-414.
Chen W, Zhang X, Ye X, Ying P (2023) Diagnostic accuracy of optic nerve sheath diameter on ultrasound for the detection of increased intracranial pressure in patients with traumatic brain injury: A systematic review and meta-analysis. Biomed Rep. 19(6):103.
Demi L, Wofram F, Klersy C et al. (2023) New International Guidelines and Consensus on the Use of Lung Ultrasound. J Ultrasound Med. 42(2):309-344.
Du J, Deng Y, Li H et al. (2020) Ratio of Optic Nerve Sheath Diameter to Eyeball Transverse Diameter by Ultrasound Can Predict Intracranial Hypertension in Traumatic Brain Injury Patients: A Prospective Study. Neurocrit Care. 32(2):478-485.
Falster C, Jacobsen N, Coman KE et al. (2022) Diagnostic accuracy of focused deep venous, lung, cardiac and multiorgan ultrasound in suspected pulmonary embolism: a systematic review and meta-analysis. Thorax. 77(7):679-689.
Fatima N, Shuaib A, Chughtai TS et al. (2019) The role of transcranial Doppler in traumatic brain injury: A systemic review and meta-analysis. Asian J Neurosurg. 14:626-33.
Fernando SM, Tran A, Cheng W et al. (2019) Diagnosis of elevated intracranial pressure in critically ill adults: systematic review and meta-analysis. BMJ. 366:l4225.
Gallardo A, Dévoli AP, Saavedra S et al. (2023) Ultrasonografia en cuidados críticos. Revista Chilena de Anestesiología. 52(1).
Gargani L, Girerd N, Platz E et al. (2023) Lung ultrasound in acute and chronic heart failure: a clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging. 24(12):1569-1582.
Goligher EC, Fan E, Herridge MS et al. (2015) Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. AJRCCM. 192:1080-1088.
Hamzaoui O, Monnet X, Teboul JL (2013) Pulsus paradoxus. Eur Respir J. 42(6):1696-705.
Imazio M, De Ferrari GM (2020) Cardiac tamponade: an educational review. Eur Heart J Acute Cardiovasc Care. 2048872620939341.
Kakkos SK, Gohel M, Baekgaard N et al. (2021) European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 61(1):9-82.
Khan F, Tritschler T, Kahn SR, Rodger MA (2021) Venous thromboembolism. Lancet (London, England). 398(10294):64-77.
Konstantinides SV, Meyer G, Becattini C et al. (2020) 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 41:543-603.
Lau YH, See KC (2022) Point-of-care ultrasound for critically ill patients: A mini-review of key diagnostic features and protocols. World J Crit Care Med. 11(2):70-84.
Lichtenstein DA (2015) BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 147(6):1659-1670.
Long B, April MD (2019) What Is the Diagnostic Accuracy of Point-of-Care Ultrasonography in Patients With Suspected Blunt Thoracoabdominal Trauma? Annals of emergency medicine. 74(3):400-402.
Martí-Mestre X, Rodríguez-Morata A, Rial-Horcajo R et al. (2023). Guía de la exploración venosa de los miembros inferiores del capítulo de diagnóstico vascular de la SEACV. Angiología. 75(1):25-42.
Martínez-Palacios K, Vásquez-García S, Fariyike OA et al. (2023) Noninvasive ICP monitoring international consensus group. Using Optic Nerve Sheath Diameter for Intracranial Pressure (ICP) Monitoring in Traumatic Brain Injury: A Scoping Review. Neurocrit Care.
Matthay MA, Arabi Y, Arroliga AC et al. (2024) A New Global Definition of Acute Respiratory Distress Syndrome. AJRCCM. 209(1):37-47.
McKaigney CJ, Krantz MJ, La Rocque CL et al. (2014) E-point septal separation: a bedside tool for emergency physician assessment of left ventricular ejection fraction. Am J Emerg Med. 32(6):493-7.
Montgomery SP, Moore B, Hampton SM et al. (2023) Optic Nerve Sheath Point of Care Ultrasound: Image Acquisition. J Vis Exp. 198:10.3791/64929.
Montorfano L, Yu Q, Bordes SJ et al. (2021) Mean value of B-mode optic nerve sheath diameter as an indicator of increased intracranial pressure: a systematic review and meta-analysis. Ultrasound J. 13(1):35.
Muñoz Rodríguez FJ (2020) Diagnosis of deep vein thrombosis. Diagnóstico de la trombosis venosa profunda. Revista clinica espanola, S0014 2565(20)30132-6.
Needleman L, Cronan JJ, Lilly MP et al. (2018) Ultrasound for lower extremity deep venous thrombosis multidisciplinary recommendations from the Society of Radiologists in Ultrasound Consensus Conference. Circulation.137:1505-15.
Nishijima DK, Simel DL, Wisner DH, Holmes JF (2012) Does this adult patient have a blunt intra-abdominal injury? JAMA. 307(14):1517-1527.
Núñez-Ramos JA, Pana-Toloza MC, Palacio-Held SC (2022) E-Point Septal Separation Accuracy for the Diagnosis of Mild and Severe Reduced Ejection Fraction in Emergency Department Patients. POCUS J. 7(1):160-165.
Parada-Gereda HM, Tibaduiza AL, Rico-Mendoza A et al. (2023) Effectiveness of diaphragmatic ultrasound as a predictor of successful weaning from mechanical ventilation: a systematic review and meta-analysis. Crit Care. 27(1):174.
Pérez-Nieto OR, Zamarron-Lopez EI, Miño-Bernal J et al. (2021) Management of Pulmonary Embolism in the Intensive Care Unit. ICU Management & Practice. 21(6):269-277.
Raboel PH, Bartek J Jr, Andresen M et al. (2012) Intracranial Pressure Monitoring: Invasive versus Non-Invasive Methods-A Review. Crit Care Res Pract. 950393.
Robba C, Taccone FS (2019) How I use Transcranial Doppler. Crit Care. 23(1):420.
Roberts ME, Rahman NM, Maskell NA et al. (2023) BTS Pleural Guideline Development Group. British Thoracic Society Guideline for pleural disease. Thorax. 78(Suppl 3):s1-s42.
Santana PV, Cardenas LZ, Albuquerque ALP (2023) Diaphragm Ultrasound in Critically Ill Patients on Mechanical Ventilation-Evolving Concepts. Diagnostics (Basel). 13(6):1116.
Savoia P, Jayanthi SK, Chammas MC (2023) Focused Assessment with Sonography for Trauma (FAST). Journal of medical ultrasound. 31(2):101–106.
Schick AL, Kaine JC, Al-Sadhan NA et al. (2023) Focused cardiac ultrasound with mitral annular plane systolic excursion (MAPSE) detection of left ventricular dysfunction. Am J Emerg Med. 68:52-58.
Stengel D, Leisterer J, Ferrada P et al. (2018) Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. The Cochrane database of systematic reviews. 12(12):CD012669.
Stengel D, Rademacher G, Ekkernkamp A et al. (2015) Emergency ultrasound-based algorithms for diagnosing blunt abdominal trauma. The Cochrane database of systematic reviews. (9):CD004446.
Vaiman M, Gottlieb P, Bekerman I (2014) Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 10:32.
Yoshida T, Yoshida T, Noma H et al. (2023) Diagnostic accuracy of point-of-care ultrasound for shock: a systematic review and meta-analysis. Crit Care. 27, 200.
Yuan X, Liu L, Chang W et al. (2021) Diagnosis Accuracy of Lung Ultrasound for ARF in Critically Ill Patients: A Systematic Review and Meta-Analysis. Front Med. 8:705960.
Zhou L, Wiley BM (2024) Current and Future Role of Ultrasonography in the Cardiac Intensive Care Unit. Critical care clinics. 40(1):15-35.